Public Citizen Comment: FCC Proposal on AI- Generated Content in Political Advertisements
Download PDF version of comment
September 19, 2024
Federal Communications Commission
Washington, D.C. 20554
Re: Comments In the Matter of Disclosure and Transparency of Artificial Intelligence- Generated Content in Political Advertisements, MB Docket No. 24-211
To the Federal Communications Commission:
Public Citizen is a national consumer rights and pro-democracy organization with more than 500,000 members and supporters. We submit these comments to support strongly the Commission’s proposed rule on disclosure and transparency of artificial intelligence-generated content in political advertisements, and to suggest some refinements.
In these comments, we make the following key points:
- Misleading artificial intelligence-generated audio and video content is proliferating in the United States and around the world and poses a serious threat to democratic integrity.
- The harms from deceptive deepfakes can be substantially mitigated or avoided through disclosures that inform viewers and listeners that they are seeing or hearing AI-generated content. A Federal Communications Commission disclosure requirement for AI-generated content is thus strongly justified by the 47 USC §303(r)’s public interest standard and easily satisfies a cost-benefit calculus.
- The threats from misleading artificial intelligence-generated audio and video content apply equally – or perhaps even more seriously – to cable operators, DBS providers and SDARS licensees and the Commission’s rule should extend to these entities.
- Disclosure should be calibrated so as not to apply to all or virtually all political advertisements. Recognizing potential ambiguity in the Commission’s proposed definition of “artificially generated content,” we propose a modest revision to the definition to clarify that the disclosure requirement would apply to content that is completely or predominantly generated by AI or significantly edited by AI tools.
- The disclosure obligation proposed by the Commission is completely compatible with the First Amendment and can withstand even heightened levels of scrutiny.
- The disclosure obligation is compatible with the Communications Act Section 315(a)’s anti-censorship provision. It is important that the Commission make a formal interpretation to this effect, because, currently, the anti-censorship provision is working to preempt state action on AI and broadcasters, as well as to impede broadcasters from requiring common sense disclosures even if they know a political ad contains deceptive AI-generated content.
Deepfakes’ Threat to Broadcast and Cable Integrity and the FCC’s Authority to Act
Extraordinary advances in artificial intelligence now provide everyone — from individuals to political candidates to outside organizations to trillion-dollar companies — with the means to produce campaign ads and other communications with computer-generated fake images, audio or video of candidates that convincingly appear to be real. These ads can fraudulently misrepresent what candidates say or do and thereby influence the outcome of an election. Already, deepfake audios can be almost impossible to detect; images are extremely convincing; and high-quality videos appear real to a casual viewer – and the technology is improving extremely rapidly.
The risks that AI-generated political ads pose to the information ecosystem are plain. First, deepfakes heighten the risk that false information or impressions will affect election outcomes. A late-breaking deepfake – for example, falsely showing a candidate making a racist statement, or slurring their words, or accepting a bribe – aired days before an election could easily sway the election’s outcome. Even if aired earlier in the election cycle, political deepfakes can leave lasting, fraudulent impressions. Deepfakes are extremely difficult to rebut, because they require a victim to persuade people not to believe what they saw or heard with their own eyes and ears. Stated differently, deepfakes appear to be “direct evidence” rather than “hearsay.” This is qualitatively different than campaign ads simply making false or misleading statements about a candidate. Because the risk they pose is categorically different from the risk posed by untrue or misleading statements about a candidate.
Second, in addition to the direct fraud they perpetrate, the proliferation of AI-generated content offers the prospect of a “liar’s dividend,” in which a candidate legitimately caught doing something reprehensible claims that authentic media is AI generated and fake.[1] It will also be possible for candidates to generate their own deepfakes to cover up their actions. Conflicting images and audios of what a candidate said or did could be used to lead a skeptical public to doubt the authenticity of genuine audio or video evidence.
Third, the stakes of an unregulated and undisclosed Wild West of AI-generated campaign communications are far more consequential than the impact on candidates; it will erode the public’s confidence in the integrity of the broadcast ecosystem itself. Congress intended FCC-licensed stations to serve the public interest by, among other things, providing them with special rights and responsibilities with respect to election communications. But if voters cannot discern reality from verisimilitude because the use of deepfakes is not disclosed, they will increasingly lose confidence in the ability of broadcast and cable TV and radio to deliver trustworthy election information to the public.
Political deepfakes are both growing in sophistication and becoming more common. Deepfakes have already influenced elections around the world. A Wired magazine database highlights examples across the globe.[2] Deepfakes are said to have impacted election results in Slovakia,[3] damaged election integrity in Pakistan[4] and spread disinformation in Argentina[5] and Mexico.[6]
In the United States, political deepfakes – of varying levels of sophistication – are also proliferating. Governor Ron DeSantis’s campaign published a deepfake image showing former President Donald Trump embracing and kissing Dr. Anthony Fauci,[7] a deepfake appeared to show Chicago mayoral candidate Paul Vallas condoning police brutality[8] and a Super PAC posted deepfake videos falsely depicting then-congressional candidate Mark Walker saying his opponent is more qualified than him.[9]
A political consultant used a deepfake version of President Biden’s voice on robocalls discouraging New Hampshire voters from turning out for the state’s primary[10] (and has now been indicted on felony charges).[11] In July 2024, Elon Musk posted a deepfake video of Vice President Harris,[12] in violation of X/Twitter’s own deepfake policy.[13] Pop star Taylor Swift has criticized deepfakes falsely depicting her as endorsing Donald Trump,[14] and deepfake celebrity endorsements of political candidates are mushrooming.[15]
Deepfakes pose special concern for communities of color and non-English speakers. There is a long and disturbing history of mass communications targeted at Black and Spanish-speaking voters that aim to discourage them from voting, using both disinformation and implicit threats. Generative AI provides these malicious actors with a new tool of targeted, fraudulent communications, for example with advertisements with misleading voting information, apparently spoken by credible figures, on Spanish-language TV and radio that may not have the same visibility as such tactics would have on English-language programming.[16]
In short, political deepfakes are a here-and-now problem, poised to become much more severe as quickly evolving generative AI technologies make deepfakes easier to produce at ever higher levels of quality. And, in the absence of regulation, deepfakes could become normalized in the public’s mind, making it much more difficult address the problem later.
The good news is, these harms are easily curable. We agree with the Commission’s conclusion in ¶14 of the Notice of Proposed Rulemaking (NPRM): If viewers and listeners are given clear and prominent disclosures that they are viewing images or listening to audio that was generated using AI technologies, they can evaluate the content appropriately and not be tricked into believing they are viewing or hearing authentic content. Generative AI technologies offer many creative benefits and are becoming increasingly enmeshed in video and audio software. The specific harm from AI-generated imagery and audio is that people may believe they are looking at authentic content when in fact it is AI-generated. Robust disclosures can largely cure this problem.
Against this backdrop, it is imperative that the FCC act. In NPRM ¶27, the Commission asks if it has the authority to adopt the proposed on-air disclosure and political file requirements for AI-generated content in political ads. We agree with the Commission’s conclusion that 47 USC §303(r)’s public interest standard provides ample authority for the proposal. The proposal will go far to ameliorate the very likely harms that will occur in the absence of regulation and serve the Commission’s mission of protecting the integrity of the broadcast system and ensuring transparency in campaign advertisements.
Similarly, in ¶22, the Commission proposes that the obligation for on-air disclosure and political file requirements for AI-generated content in political ads be applied to cable operators, DBS providers and SDARS licensees. We agree with this conclusion, which follows logically from the existing application of political programming and sponsorship requirements to these entities. Indeed, because these entities often engage in more narrowcasting than TV and radio broadcasters, it is arguably more important that the disclosure and record-keeping requirements apply to them; narrowcasting raises the prospect of more targeted fraudulent and deceptive deepfakes, which may seem more effective and more likely to evade detection, including especially when targeting non-English speaking audiences.
In ¶36, the Commission asks about costs and benefits. For the reasons stated by the Commission, we agree that the costs would be small. None of the elements of the proposed rule – a simple request requiring no additional data gathering by the advertiser, an entry into the political file and a straightforward, pre-established disclosure – should entail more than very modest costs. By contrast, for the reasons stated by the Commission and elaborated in this comment, we believe the benefits would be substantial and consequential.
In ¶37, the Commission asks about impacts on digital equity. For the reasons explained above about potential AI-generated advertising targeted at communities of color and especially non-English speaking audiences, we believe the proposal would meaningfully advance digital equity and inclusion.
Definition and Scope of Disclosure
The Commission defines artificial intelligence in NPRM ¶11, relying on the definition provided in President Biden’s AI Executive Order, which itself draws from a prior statutory definition. Recognizing there is no precise definition of AI possible, we believe this definition is appropriate.
In ¶12, the Commission proposes to define “AI-generated content” as “an image, audio, or video that has been generated using computational technology or other machine-based system that depicts an individual’s appearance, speech, or conduct, or an event, circumstance, or situation, including, in particular, AI-generated voices that sound like human voices, and AI-generated actors that appear to be human actors.”
The definition of “AI-generated content” is extremely important in the context of the rule, because it defines the scope of political advertisements for which a disclosure must be made. While it is vital that the Commission mandate disclosures of AI-generated content, the benefits of those disclosures will be lost if the disclosure requirement is overbroad. As AI editing tools become commonplace, we may soon reach a position where almost all ads could be said to contain AI-generated content.
While we believe the Commission’s proposed definition could fairly be read to avoid this result, we recommend a refinement to avoid ambiguities that may lead to an overbroad definition. We recommend modifying the definition of “AI-generated content” to cover only content that has been primarily generated or significantly edited by AI technologies. We propose specific language further below.
Examining the policies of major social media platforms that have already been forced to address this issue provides some useful examples of well-developed, experience-based, generative AI labeling policies.
TikTok: TikTok requires the labeling of videos that are “completely generated or significantly edited by AI.”[17] TikTok elaborates on this standard as follows:
We consider content that’s significantly edited by AI as that which uses real images/video as source material, but has been modified by AI beyond minor corrections or enhancements, including synthetic images/video in which:
- The primary subjects are portrayed doing something they didn’t do, e.g. dancing.
- The primary subjects are portrayed saying something they didn’t say, e.g. by AI voice cloning; or
- The appearance of the primary subject(s) has been substantially altered, such that the original subject(s) is no longer recognizable, e.g. with an AI face-swap.
Twitter/X: Twitter/X’s policy on generative AI is included in its overall policy on misleading media. X requires labeling or removal of material “that is significantly and deceptively altered, manipulated, or fabricated.”[18] X elaborates on these standards by listing relevant factors to determine if media meets these criteria:
- Whether media have been substantially edited or post-processed in a manner that fundamentally alters their composition, sequence, timing, or framing and distorts their meaning;
- Whether there are any visual or auditory information (such as new video frames, overdubbed audio, or modified subtitles) that has been added, edited, or removed that fundamentally changes the understanding, meaning, or context of the media;
- Whether media have been created, edited, or post-processed with enhancements or use of filters that fundamentally changes the understanding, meaning, or context of the content; and
- Whether media depicting a real person have been fabricated or simulated, especially through use of artificial intelligence algorithms.
Meta/Facebook/Instagram: Meta requires labeling of AI-generated content.[19] It has recently updated its policy to distinguish between content that is generated with AI and content that was edited using AI tools. This reflected the company’s experience that using modest retouching AI tools would lead content to be labeled as “Made with AI,” which created user confusion and dissatisfaction due to perceived over-labeling.
Meta’s recently revised policy requires prominent labeling only for content that was generated by AI. When content is modified or edited by AI tools, the information is still available for users, but not prominently displayed:
For content that we detect was only modified or edited by AI tools, we are moving the “AI info” label to the post’s menu. We will still display the “AI info” label for content we detect was generated by an AI tool and share whether the content is labeled because of industry-shared signals or because someone self-disclosed.
Youtube: YouTube requires labeling of content that was “meaningfully altered or synthetically generated when it seems realistic.”[20] This standard is further elaborated:
To help keep viewers informed about the content they’re viewing, we require creators to disclose content that is meaningfully altered or synthetically generated when it seems realistic.
Creators must disclose content that:
- Makes a real person appear to say or do something they didn’t do
- Alters footage of a real event or place
- Generates a realistic-looking scene that didn’t actually occur.
This could include content that is fully or partially altered or created using audio, video or image creation or editing tools.
Altogether, the lessons from the platforms’ AI disclosure policies are:
- Avoid overbroad labeling from use of AI editing tools.
- Require labeling based on a standard around concepts like: “completely generated” by AI and “significantly edited or altered” by AI.
- Elaborate the overarching principle with more specific sub-principles, such as showing a person saying or doing something they did not say or do.
Taking these lessons into account, we propose the following definition of “Artificial Intelligence-Generated Content” for purposes of the rule:
“Artificial Intelligence-Generated Content is defined for purposes of this section as an image, audio, or video that has been completely or primarily generated or significantly edited using computational technology or other machine-based systems that depict an individual’s appearance, speech, or conduct, or an event, circumstance, or situation, including an image, audio or video in which:
- The primary subjects are portrayed doing something they didn’t do or in realistic looking scenes that did not occur or which have been substantially altered;
- The primary subjects are portrayed saying something they didn’t say;
- The appearance of the primary subjects has been substantially altered in a realistic-looking manner (e.g., a person is realistically depicted wearing a T-shirt with a slogan that they did not wear).”
Implementation Issues
The Commission requests comment on a number of specific implementation issues.
In NPRM ¶16, the Commission asks about the timing of required disclosures. Disclosures should be required immediately prior to/at the start of a political ad. An upfront disclosure is necessary so that viewers and listeners have the appropriate context in which to assess AI-generated content. An after-the-fact disclosure does not empower viewers and listeners in real time to evaluate the content with knowledge that it was AI generated. An after-the-fact disclosure is also easier for viewers or listeners to miss or mentally tune out.
In ¶17, the Commission raises the scenario of a broadcaster being informed by a credible third party that an ad was generated with artificial intelligence where there was no previous disclosure by the advertiser. Where the credible third party’s information is compelling (for example, where a depicted person attests that they did not say or do the things the ad realistically shows them as saying or doing), the broadcaster should attach an AI disclosure. This disclosure should come at the beginning of the ad, for the same reasons that all such disclosures should be made at the start of ads. If the credible third party’s information raises legitimate questions but falls short of compelling evidence, the broadcaster should immediately follow up with the advertiser.
In ¶21, the Commission asks about syndicated programming. We recommend a multi-tiered response. Broadcasters should inform network and syndication partners of the broadcasters’ duty to include disclosures for AI-generated content in political ads. They should inquire of their partners if any such ads are included in their programming, as they would inquire of direct advertisers. This inquiry should occur at the start of each television season or calendar year; but, more importantly, it should occur within a window near every primary and general election, say each month within 120 days before the election. Because each inquiry involves de minimis effort, frequent inquiries would not be burdensome.
The case of syndicated and network programming creates longer chains of responsibility and makes it particularly important that robust systems are in place for broadcasters to insert disclosures when they receive compelling information from credible third parties that an ad was generated with artificial intelligence where there was no previous disclosure by the advertiser.
First Amendment Considerations
Public Citizen agrees with the Commission’s tentative conclusion that the First Amendment does not bar the imposition of disclosure requirements on broadcast and other licensed facilities to combat the improper use of AI in political advertising. See NPRM ¶ 29. As the NPRM explains, different levels of First Amendment scrutiny apply in different contexts, but all the various tests examine the nature of the governmental interests at stake and the means used to achieve them. We agree that the proposed rules would be consistent with the First Amendment even under heightened forms of scrutiny.
At the outset, the Supreme Court has recognized that the government has a legitimate interest in protecting the public from being misled and that disclosure requirements tailored to advance that interest can survive “exacting scrutiny.” Citizens United v. FEC, 558 U.S. 310, 366–67 (2010) (citing Buckley v. Valeo, 424 U.S. 1, 64 (1976), and McConnell v FEC, 540 U.S. 93, 201 (2003)). In Citizens United, for instance, the Court upheld a requirement that televised electioneering communications “include a disclaimer” identifying the name and address of the person responsible for the communication and explaining that the advertisement was not funded by a candidate or candidate committee. Id. at 366. The Court explained that the requirement advanced the government’s interests in “provid[ing] the electorate with information,” “insur[ing] that the voters are fully informed about the person or group who is speaking,” and “making clear that the ads are not funded by a candidate or political party.” Id. at 368 (cleaned up). The Court rejected the argument that the disclosure requirement was underinclusive because it applied only to broadcast advertising and communications on other media. Id. at 368. And although the Court recognized that “[d]isclaimer and disclosure requirements may burden the ability to speak,” it observed that a disclosure requirement “impose[s] no ceiling on campaign-related activities,” … and “do[es] not prevent anyone from speaking.’” Id. at 366.
The Court also applied “exacting scrutiny” to uphold a requirement that signatories on a referendum be disclosed. Doe v. Reed, 561 U.S. 186, 196 (2010). The Court explained that the state’s “interest in preserving the integrity of the electoral process is undoubtedly important,” id. at 197, and “not limited to combating fraud,” id. at 198. The Court also concluded that the disclosure requirement was a valid means of advancing that interest because it “can help cure inadequacies” in other methods used to protect against invalid signatures. Id. at 198. See also Nat’l Ass’n of Manuf. v. Taylor, 582 F.3d 1, 11 (D.C. Cir. 2009) (holding that law requiring disclosure of lobbying information survived strict scrutiny).
As the NPRM observes, the government has a vital interest in ensuring that broadcasters “assume responsibility for all material which is broadcast through their facilities” and “take reasonable measure to address any false, misleading, or deceptive matter.” NPRM ¶ 30. That is especially true for election-related advertising, where Congress has imposed important public-interest responsibilities on broadcast licensees. Id. In the context of political advertising, the government and the public have an especially strong interest in prohibiting the use of AI-generated content to disseminate “deceptive, misleading, or fraudulent information to voters.” Id. When a candidate makes a deceptive verbal representation about an opponent, it is possible to mitigate the impact of the misrepresentation by persuasively exposing it as untrue. See United States v. Alvarez, 567 U.S. 709, 727 (2012) (plurality opinion) (“The remedy for speech that is false is speech that is true.”). AI technology, however, allows advertising to be created that deceptively appears to the public to be authentic evidence of a claim for or against a candidate or an issue.
Absent a disclosure requirement, such advertising can manipulate public opinion illegitimately, precisely because it is far more difficult to expose through counter-speech. Cf. FTC v. Colgate-Palmolive Co., 380 U.S. 374, 386 (1965) (recognizing that a “representation to the public” that “a viewer is seeing” evidence of a claim “for himself” may be a “false” representation that influences consumer purchasing decisions). Informing the public that political advertising is AI-generated ensures that they have the information they need to correctly “evaluate the arguments to which they are being subjected.” First Nat’l Bank of Bos. v. Bellotti, 435 U.S. 765, 792 n.32 (1978). “[D]isclosure is a less restrictive alternative to more comprehensive regulations of speech,” Citizens United, 558 U.S. at 369, which in this case might otherwise be a ban on advertisements primarily generated or substantially edited by AI. The First Amendment does not prevent the Commission from requiring disclosures designed to avoid misleading political advertising on a medium charged with operating in the public interest. See FCC v. League of Women Voters of Cal., 468 U.S. 364, 378 (1984) (recognizing that broadcasters “bear the public trust”).
Content-Neutral Disclaimers and Section 315(a)
In NPRM footnote 54, the Commission tentatively concludes that content-neutral disclaimers of the sort proposed by the Commission are consistent with section 315(a) of the Communications Act and do not constitute censorship. The Bureau’s precedent and common sense both support this conclusion, and we agree with the Commission’s tentative conclusion. The proposed disclaimers do not prevent any ad from airing, and they do not discriminate based on viewpoint; they merely provide transparency to viewers and listeners and prevent fraud and deception that is otherwise significantly incurable by voters or negatively impacted candidates themselves.
The Section 315(a) issue is crucial because it underscores that the FCC effectively is already regulating related to the use of artificial intelligence in political ads. More than 20 state legislatures have passed legislation to regulate and require disclosure of political deepfakes.[21] Most states and commentators believe that Section 315(a) exercises a preemptive force against such state regulation and most or all of the state laws exempt broadcasters from coverage. The broadcasters believe that Section 315(a) prevents them from requiring disclosures on deceptive AI-generated ads even if they are certain the ads were generated by AI.
Thus, clarifying that content-neutral disclaimer requirements are consistent with Section 315(a) is necessary and important independent of any other regulatory action the Commission takes related to the use of artificial intelligence in political ads. Such a clarification would free states to regulate the use of artificial intelligence in political ads consistently across all platforms, as they are now constrained from doing; and it would enable the broadcasters to exercise common sense and require disclosures on content that they know is primarily AI-generated or AI-meaningfully edited.
If the Commission is not able to move expeditiously – before the 2024 election — to finalize this proposed rule, we encourage the Commission to issue a standalone interpretive finding that content-neutral disclaimers relating to the use of AI in political ads are consistent with section 315(a) of the Communications Act and do not constitute censorship.
Conclusion
We applaud the Commission for proactively taking up this issue and helping protect democracy and reduce distrust with broadcast material. The challenges of AI-generated political ads are fast rushing at America and the world, and we urge the Commission to move as quickly as possible to refine and finalize its proposal.
Sincerely,
Robert Weissman
Co-president, Public Citizen
1600 20th St., NW
Washington, D.C.
(202) 588-1000
[1] Bryan McKenzie, “Is that real? Deepfakes could pose danger to free elections,” UVA Today, August 24, 2023,
https://news.virginia.edu/content/real-deepfakes-could-pose-danger-free-elections#:~:text=A%20deepfake%20is%20a%20computer,to%20entertainment%2C%20hoaxes%20to%20harassment. See also: Josh Goldstein and Andrew Lohn, “Deepfakes, Elections and Shrinking the Liar’s Dividend,” Brennan Center, January 23, 2024, https://www.brennancenter.org/our-work/research-reports/deepfakes-elections-and-shrinking-liars-dividend.
[2] The Wired AI Elections Project, May 30, 2024, https://www.wired.com/story/generative-ai-global-elections.
[3] Olivia Solon, “Trolls in Slovakian Election Tap AI Deepfakes to Spread Disinfo,” Bloomberg, September 29, 2023, https://www.bloomberg.com/news/articles/2023-09-29/trolls-in-slovakian-election-tap-ai-deepfakes-to-spread-disinfo; Morgan Meaker, “Slovakia’s Election Deepfakes Show AI is a Danger to Democracy,” Wired, October. 3, 2023), https://www.wired.co.uk/article/slovakia-election-deepfakes.
[4] Nilofar Mughal, Deepfakes, Internet Access Cuts Make Election Coverage Hard, Journalists Say, VOA, February 22, 2024, https://www.voanews.com/a/deepfakes-internet-access-cuts-make-election-coverage-hard-journalists-say-/7498917.html.
[5] Jack Nicas and Lucía Cholakian Herrera, “Is Argentina the First AI Election?” New York Times, November 15, 2023, https://www.nytimes.com/2023/11/15/world/americas/argentina-election-ai-milei-massa.html.
[6] Emily Kohlman, “Deepfakes, Identity Politics, and Misinformation Campaigns Target the Mexican Election,” Blackbird.AI, https://blackbird.ai/blog/mexico-election-deepfakes-identity-politics-misinformation-disinformation.
[7] Nicholas Nehamas, “DeSantis campaign uses apparently fake images to attack Trump on Twitter, New York Times, June 8, 2023, https://www.nytimes.com/2023/06/08/us/politics/desantis-deepfakes-trump-fauci.html.
[8] Megan Hickey, “Vallas campaign condemns deepfake posted to Twitter,” CBS News, February 27, 2023,
https://www.cbsnews.com/chicago/news/vallas-campaign-deepfake-video.
[9] Danielle Battaglia, “‘Deepfake’ videos target Mark Walker in NC congressional campaign,” The News and Observer, February 29, 2024, https://www.newsobserver.com/news/politics-government/election/article286043906.html.
[10] Alex Seitz-Wald and Mike Memoli, “Fake Joe Biden robocall tells New Hampshire Democrats not to vote Tuesday,” NBC News, January 22, 2024, https://www.nbcnews.com/politics/2024-election/fake-joe-biden-robocall-tells-new-hampshire-democrats-not-vote-tuesday-rcna134984.
[11] Ross Ketschke, “Political consultant indicted for AI robocalls with fake Biden voice made to New Hampshire voters,” WMUR9, May 23, 2024, https://www.wmur.com/article/steve-kramer-ai-robocalls-biden-indictment-52224/60875422.
[12] Mirna Alsharif, Alexandra Marquez and Austin Mullen, “Elon Musk retweets altered Kamala Harris campaign ad,” NBC News, July 28, 2024, https://www.nbcnews.com/news/us-news/elon-musk-retweets-altered-kamala-harris-campaign-ad-rcna163985.
[13] “Letter to Elon Musk and X,” Public Citizen, July 29, 2024, https://www.citizen.org/article/letter-to-elon-musk.
[14] https://www.instagram.com/taylorswift/p/C_wtAOKOW1z/?hl=en.
[15] Fake celebrity endorsements become latest weapon in misinformation wars, sowing confusion ahead of 2024 election
[16] Astrid Galván, “First AI election renews battle against Spanish-language misinformation,” Axios, February 22, 2024, https://www.axios.com/2024/02/22/misinformation-generative-ai-deepfakes-spanish-language; “Comments on Public Citizen’s Petition,” Unidos, October 16, 2023, https://unidosus.org/wp-content/uploads/2023/10/unidosus_commentsonpubliccitizenspetitionforrulemakingonartificialintelligence_incampaignads_docket2023%E2%80%9313.pdf.
[17] “About AI-Generated Content,” TikTok, https://support.tiktok.com/en/using-tiktok/creating-videos/ai-generated-content.
[18] “Synthetic and Manipulated Media Policy,” X, https://help.x.com/en/rules-and-policies/manipulated-media.
[19] Monika Bickert, “Our Approach to Labeling AI-Generated Content and Manipulated Media,” Meta, https://about.fb.com/news/2024/04/metas-approach-to-labeling-ai-generated-content-and-manipulated-media.
[20] “Disclosing Use of Synthetic or Altered Content,” Youtube, https://support.google.com/youtube/answer/14328491?hl=en.
[21] “Tracker: State Legislation on Deepfakes in Elections,” Public Citizen, https://www.citizen.org/article/tracker-legislation-on-deepfakes-in-elections.
Stay Updated on Public Citizen
Follow Public Citizen
Support Our Work
Opposition to Congressional Anti-ESG bills from National Association of Counties
Download PDF version of comment
The National Association of Counties (NACo) recently adopted a resolution opposing Congressional “Anti-ESG” legislation such as H.R. 4790 which would: “restrict and eliminate local authority regarding pensions, municipal bonds, and government funds” and “threaten county governments’ ability to deliver services to meet their own communities’ needs by restricting access to essential data and regulating local governments.” The NACo resolution and a cover letter from Coconino County Treasurer Sarah Benatar available to download by clicking here.
Stay Updated on Public Citizen
Follow Public Citizen
Support Our Work
Public Citizen letter to House on anti-ESG bills
Download PDF version of comment
September 17, 2024
The Honorable Mike Johnson
Speaker of the House
United States House of Representatives
Washington, DC 20515
The Honorable Hakeem Jeffries
Minority Leader
United States House of Representatives
Washington, DC 20515
Vote NO on anti-ESG, bank capital bills
Dear Speaker Johnson and Minority Leader Jeffries:
On behalf of more than 500,000 members and supporters of Public Citizen[1], we oppose a suite of bills scheduled for House consideration this week that attack the ability of investors to improve their financial decisions with key environmental, social and governance (ESG) information. Promoted as “anti-woke” measures, they represent fossilized thinking at the behest of the fossil fuel industry. They defy widespread public interest in ESG issues, as polls affirm.[2] Public Citizen commissioned a poll and found that voters oppose federal limits on information available to the public, including institutional and individual investors. Voters support representatives who favor corporate disclosures on ESG metrics.[3] Separately, another dangerous measure attached to the anti-ESG measures attacks bank capital safety reforms. We ask the House to Vote No on all these bills.
HR 4790, The Guiding Uniform and Responsible Disclosure Requirements and Information Limits Act, would cede to corporations the final decision on what it considers material information, which is information that may affect investment decisions. This functionally renders all disclosures discretionary, retreating nearly to the era before the federal securities laws were approved in the 1930s to prevent another 1929-level market crash. The bill also creates a Public Company Advisory Committee composed exclusively of executives to counsel the Securities and Exchange Commission (SEC). Unlike the SEC’s other advisory committees, which already include corporate representatives, taxpayers would foot the bill for executive travel and other expenses. Corporate executives, who are handsomely paid, already enjoy ample voice in SEC policymaking through the bi-partisan commission make-up, public comment on rules, other advisory committees and an open door for meetings, not to mention an army of lobbyists.
The bill also requires the SEC to list all requirements for “non-material” information that must be disclosed and bars private litigation for corporate failure to disclose this information. Some information, perhaps such as the name of the corporate secretary, is necessary for administrative purposes and thus such a list would be nonsensical.
HR 4767, Protecting Americans’ Retirement Savings from Politics Act, and HR 4655, the Businesses Over Activists Act, would eviscerate shareholder democracy. (These bills may be incorporated as titles in HR 4790.) Specifically, HR 4767 would eliminate the ability of shareholders to file ESG related resolutions. It also restricts the ability of proxy advisory firms to consult with institutional investors on the merits of specific shareholder resolutions by requiring extensive disclosures and opportunities for management censorship. This is a hypocritical proposal from the same Republicans who generally decry management disclosure requirements yet now attempt to stifle potential dissent with what they would otherwise characterize as regulatory burden.
More bluntly, HR 4655 eliminates the power of the SEC to enforce federal rights of shareholders to file resolutions altogether. Shareholder resolutions serve to reform corporate governance and empower company owners to assert their interests as a check against the potentially self-interested actions of managers.[4] Notable progress through the suffrage of shareholder resolutions include the establishment of annual board elections; requiring a chair be an individual who is not also the CEO; bridling excessive compensation; and promoting reports on such vital issues as a company’s contribution to climate change. Management may bristle when directed to improve behavior. But capitalism pivots on the ability of those providing investment funding to exercise their ownership rights. These bills serve only to entrench management, fatten C-suite compensation, and buttress destructive corporate behavior.
HR 4823, American Financial Institution Regulatory Sovereignty and Transparency Act of 2023, would eliminate the position of “vice chair for supervision” at the Federal Reserve Board of Governors. (As with HR 4767 and HR 4655, this may be included as a title in HR 4790 even though it does not relate to shareholder suffrage.) The bill also requires all financial regulatory agencies to file extensive analysis of proposed rules with Congress. Finally, the bill bars banking regulators from meeting with an international organization on the “topic of climate-related financial risk” unless they file a report with Congress about any such organization, including its funding sources.
This provision clearly reflects the House Financial Services Committee Republicans’ year-long assault against the current efforts of financial regulators to finalize improved capital safety requirements for the largest banks. Michael Barr serves as the Fed’s Vice Chair of Supervision and leads the effort to improve safety, and stripping his title is little more than a juvenile taunt. Ultimately, these provisions do nothing to address the underlying issues in the financial sector. Capital, namely the difference between the value of the bank’s assets and liabilities, constitutes a primary bulwark of safety. When this difference is greater, when the bank maintains greater capital, it can sustain losses and continue to operate. When this difference is smaller, when capital is less, losses can lead to insolvency, that is, liabilities exceed assets. With the largest bank, insolvency then leads to a taxpayer bailout, as witnessed in the 2008 financial crisis.[5] Perversely, bank managers prefer to operate with as little capital as possible, in part, because it boosts C-suite pay. Most executive pay turns on the stock price. Stock prices rise with a better return on equity capital. The simplest way to improve this ratio is to reduce the denominator, namely equity capital; the same income return is allocated to fewer shares. But that’s hardly a foundation for a sound banking sector. American financial stability should not be sacrificed at the altar of elite remuneration.
HR 5339, the RETIRE Act, introduces a new concept for investment managers who provide fiduciary services to pension and other investment funds: fiduciaries would be required to make investment decisions based exclusively on “pecuniary” factors. The bill effectively prevents these investment managers from considering collateral factors, such as an investment in a firm that can promote employment where the plan beneficiaries reside, or one that provides clean energy to the community.
As noted, Americans oppose policies such as these, as affirmed by polls. This slate of Republican-led bills services industry sponsors, not the public. These bills will receive no attention in the Senate, but it is still critical for Members to demonstrate strong opposition on the House floor. Behind the anti-ESG movement are fossil fuel firms and other extreme right groups.[6] [7] Behind the attack on bank capital safety standards are pay-bloated bank executives The American public and public policy must not be subordinated to these destructive interest groups.
For questions, please contact Jon Golinger at jgolinger@citizen.org, and/or Bartlett Naylor at bnaylor@citizen.org.
Sincerely,
Public Citizen
[1] Public Citizen is a nonprofit consumer advocacy organization with members in all fifty states. Public Citizen regularly appears before Congress, administrative agencies, and courts to support the enactment and enforcement of laws protecting consumers, workers, and the general public.
[2] ROKK Solutions and Penn State University’s Center for the Business of Sustainability, Navigating ESG in the New Congress, (2021) https://rokksolutions.com/wp-content/uploads/2022/12/Navigating-ESG-in-the-new-Congress.pdf.
[3] Lake Research Partners, Survey Findings, (Dec. 7, 2023) https://www.citizen.org/wp-content/uploads/memo.Public-Citizen.2023.12.07.pdf.
[4] Sanford Lewis, Shareholder Rights: Assessing the Threat Environment, Harvard Law School Forum on Corporate Governance (August 21, 2023)
[5] Bartlett Naylor, TOO Big, Public Citizen (2016) https://www.citizen.org/wp-content/uploads/toobig.pdf
[6] Kate Aronoff , The Deranged Demands of the “Anti-ESG” Movement New Republic (August 29, 2022) https://newrepublic.com/article/167550/desantis-anti-esg-movement
[7] Julie Bykowicz & Angela Au-Yeung, Conservatives Have a New Rallying Cry: Down With ESG, The Wall Street Journal, (Feb. 26, 2023) https://www.wsj.com/articles/conservatives-have-a-new-rallying-cry-down-with-esg-2ef98725.
You might be interested in
Stay Updated on Public Citizen
Follow Public Citizen
Support Our Work
Tracker: State Legislation to Protect Election Officials
Download PDF version of comment
In recent years, election workers and officials have been the target of ongoing threats and harassment.
The table below illustrates where states have passed or are in the process of debating legislation to protect election officials, as well as states that already had protections in their code.
Bills Passed January, 2022 – Present
| STATE | BILL | DESCRIPTION | STATUS | |
| Alabama | HB 100 | Establishes increased penalties for a crime committed against an election official that is motivated by an individual's role as an election official. This bill would also establish that a felony committed against an election official which is motivated by an individual's role as an election official is a crime of moral turpitude | Enacted in 2024 | |
| Arizona | SB-1061 | Addresses doxxing & Allows public officials, including election workers, to have their personal information removed from the public record if they believe that their life or safety is in danger | Enacted in 2023 | |
| California | SB-485 | Makes it a crime to interfere with the officers holding an election or conducting a canvass, as to prevent the election or canvass from being fairly held and lawfully conducted, or with the voters lawfully exercising their rights of voting at an election | Enacted in 2023 | |
| California | SB-1131 | Allows election workers to keep their personal information confidential and eliminates the requirement to post the names of the precinct board members | Enacted in 2022 | |
| Connecticut | HB 5498 | Makes it a crime to harasses or intimidate, or attempt to harass or intimidate, any election worker in the performance of any duty, including disclosure of personal information | Enacted in 2024 | |
| Colorado | HB-22-1273 | Makes it a crime to interfere with an election worker, or to threaten or retaliate against them; defines election worker; makes it a crime to knowingly dox an election official, and includes a confidentiality program for getting information removed | Enacted in 2022 | |
| Indiana | SB-170 | Makes it a felony to take certain actions: (1) for the purpose of influencing an election worker; (2) to obstruct or interfere with an election worker; or (3) that injure an election worker. | Enacted in 2024 | |
| Maine | HB-1821 | Provides standardized training for election officials, and makes it a crime to intentionally interfere by force, intimidation, or violence in the work of an election official or someone perceived to be an election official while they are performing their duty; provides reporting requirements for threats and harassment | Enacted in 2022 | |
| Maryland | HB 585/ SB 480 | Makes it a crime to threaten or coerce an election official or to dox an election official | Enacted 2024 | |
| Michigan | H-4129 | Makes it a crime to intimidate an election official, with the specific intent of interfering with the performance of that election official’s election-related duties | Enacted in 2023 | |
| Minnesota | H-3 | Makes it a crime to intimidate an election worker, interfere with election administration, dox an election worker, or tamper with voting equipment | Enacted in 2023 | |
| Minnesota | HF-1830 | Fully funds election security needs and codifies best practices to protect voting systems from breach | Enacted in 2023 | |
| Montana | SB-61 | Makes it a crime to intimidate an election worker, interfere with election administration, dox an election worker, or tamper with voting equipment | Enacted in 2023 | |
| Nevada | SB-406 | Provides definition of election official and makes it a crime to interfere with their duties | Enacted in 2023 | |
| New Hampshire | SB-405 | Makes it a crime to threaten, coerce, intimidate or use any violence against an election worker while they are performing their duties or as retaliation; makes it a crime to dox an election worker | Enacted in 2022 | |
| New Mexico | SB-43 | Makes it a crime to intimidate, coerce, or threaten an election worker; makes it a crime for an election official to knowingly fail to perform their duties | Enacted in 2023 | |
| North Dakota | SB-2292 | Makes it a crime to intimidate an election official for the purpose of impeding or preventing the free exercise of the elective franchise or the impartial administration of the election or Election Code | Enacted in 2023 | |
| Oklahoma | SB-481 | Makes it a crime to "cause a disturbance, breach the peace, or obstruct a qualified elector or a member of the election board on the way to or at a polling place" | Enacted in 2023 | |
| Oregon | HB-4144 | Makes it a crime to dox an election official. | Enacted in 2022 | |
| Vermont | SB-265 | Creates crime of harassment and aggravated harassment of an election worker; establishes a confidentiality program for election workers to have their information removed from public record | Enacted in 2022 | |
| Virginia | SB-364 | Makes attacks on election workers a hate crime; provides civil remedy for threats and harassment; makes it a crime to hinder the administration of election; shields providers from liability who act to protect election workers; allows election workers to hide their personal information. | Enacted in 2024 | |
| Washington | SB-5628 | Makes it a crime to terrify, intimidate, or unlawfully influence the conduct of a candidate for public office, a public servant, an election official, or a public employee | Enacted in 2022 | |
| Washington | HB 1241 | Modifies crimes of harassment and cyber harassment to cover conduct that is lewd or threatens bodily injury, property damage, confinement, or other malicious threats.Increases penalty for harassment of an election official to a class C felony; Allows election officials who are harassed to apply for the address confidentiality program. | Enacted in 2024 |
Previously Existing Protections for Election Officials*
| STATE | BILL | DESCRIPTION |
| Alaska | AS 15.56.060(a) | Makes it a crime to induce or attempt to induce an election official to fail in the official's duty by force, threat, intimidation, or offers of reward |
| Delaware | Title 15 § 5118 | Makes it a crime to attempt to molest, disturb or prevent the election officers from proceeding regularly with any general or special election |
| Georgia | Georgia Code § 21-2-566 | Criminalizes the use or threat of violence in a manner that would prevent a poll officer from the execution of his or her duties, or materially interrupts or improperly and materially interferes with the execution of a poll officer's duties |
| Louisiana | RS 14:122 | Protects election officials from violence or threats interfering with their position, employment or duties |
| North Carolina | G.S. 163-275 (11) | Criminalizes the intimidation or attempted intimidation of any chief judge, judge of election or other election officer in the discharge of duties in the registration of voters or in conducting any primary or election |
| Pennsylvania | Title 18 § 4702 | Makes it a crime to threaten unlawful harm to any person with intent to influence his decision, opinion, recommendation, vote or other exercise of discretion as a public servant, party official or voter |
| Pennsylvania | 25 Pa. Stat. § 3527 | Makes it a crime to attempt to prevent any election officers from holding any primary or election, or threaten any violence to any such officer. |
| Utah | § 20A-1-603 | Makes it a crime to interfere in any manner with any election official in the discharge of the election official's duties |
| Virginia | § 24.2-1000 | Criminalizes bribery, intimidation, threats, coercion, or other means in violation of the election laws to hinder the officer. |
| West Virginia | §3-9-10 | States that any person who prevents or attempts to prevent any officer whose duty it is by law to assist in holding an election, or in counting the votes cast, is guilty of a misdemeanor |
| Wyoming | 22-26-111 | Makes it a crime to intimidate an election official or elector for the purpose of impeding or preventing the free exercise of the elective franchise or the impartial administration of the Election Code |
Bills Introduced 2022 – Present
| STATE | BILL | DESCRIPTION | STATUS |
| Arizona | SB 1517 | Makes doxxing election workers illegal and extends existing definition of election officer to volunteer pollworkers. | Introduced in 2024 |
| Arizona | SB 1518 | Makes threatening, coercion, intimidation, retaliation, obstruction of election workers a crime and allows for civil action as an additional remedy. | Introduced in 2024 |
| Florida | H-721 / S-562 | Makes it a crime to intimidate, threaten, coerce, or harass an election worker with the intent to impede their duty, or as retaliation for their work as an election worker | Introduced in 2024 |
| Hawaii | HB1786 | Makes it a crime to harasses or intimidate, or attempt to harass or intimidate, any election worker in the performance of any duty, including disclosure of personal information. allocates funds. | Introduced in 2024 |
| Illinois | HB4827 | Makes it a crime to, impede, threaten or , intimidate. an election worker while on duty. Prohibits doxxing election workers. | Introduced in 2024 |
| Iowa | HF 2498 | Makes it a crime to intentionally hinder, interfere with, or prevent an election official in the performance of the election official’s duties: dox an election official or their family that poses an imminent and serious threat; Intentionally alter or damage any computer software or any physical part of voting equipment; or create or disclose an electronic image of the hard drive of a voting system | Introduced in 2024 |
| Kansas | HB-2190 | Prohibits threatening, intimidating, hindering, etc. an election worker on duty or in retaliation for their works. Makes it a crime to not comply with voter registration regulation. | Introduced in 2023 |
| Massachusetts | SB-1013 | Makes it a crime to threaten, intimidate, coerce, or harass an election worker while they are conducting their duties, or in retaliation for the actions of an election worker; makes it a crime to dox an election worker | Introduced in 2023 |
| Missouri | HB2140 | Makes it a crime to tamper with, harass, or intimidate an election official; makes it a crime to dox an election official | Introduced in 2023, Re-Introduced in 2024 |
| Nebraska | LB-1390 | Makes it a crime to dox, obstruct, hinder, assault, bribe, solicit, threaten, harass, eject, remove, molest, or interfere with election workers. Creates report of all reported threats and harassment of election workers | Introduced in 2024 |
| New Hampshire | HB 1364 | Makes it a crime to threaten or intimidate an election official in retaliation against the official on account of the official's performance of the official's duties; makes it a crime to dox an election official; prevents election officials from tampering with the voting system, and requires that voting systems are protected by key card access | Introduced in 2024 |
| New Jersey | A4083 / S3009 | "John R. Lewis Voter Empowerment Act of New Jersey" creates sweeping voting rights reforms; makes it a crime to engage in acts of intimidation, deception, violence or restraint, or obstruction that affects the right of voters to access the elective franchise or the performance of official duties by election workers. | Introduced in 2024, Active |
| New Jersey | S-167 / A-337 | Makes it a crime to intimidate, threaten or coerce any election official or election worker in the discharge of their duties; prevents doxxing of an election official; allows an election official to make their information private; mandates audits on election machines | Introduced in 2022, Re-Introduced in 2024, Active |
| New York | AB-4759 | Makes it a crime to intimidate or interfere with, or attempt to intimidate or interfere with, the ability of any person or any class of persons to vote or qualify to vote, or a poll watcher, or any legally authorized election official, in any primary, special, or general election | Introduced in 2023, Active |
| North Carolina | SB-313/ HB-372 | Expands the crime of threatening an election official; allows a civil remedy and compensation in addition to the existing criminal punishments; creates crime of threatening and intimidating voters and of refusal to certify an election; creates guidelines for audits of elections | Introduced in 2023, Active |
| Ohio | SB-173 | Allows election officials to have their private information kept confidential, adds them to current statues which protect firefighters and police officers; may not apply to part time election workers | Introduced in 2023, Active |
| Rhode Island | H7447 | Extends the crime of making a threat to take the life of, or to inflict bodily harm upon, a public official to election workers and poll workers. | Introduced in 2024, Active |
| South Carolina | H 5006 | Makes it a crime to harasses or intimidate, or attempt to harass or intimidate, any election worker in the performance of any duty, including disclosure of personal information. Makes doxxing election workers a crime. Makes tampering with election systems a crime. | Introduced in 2024 |
| South Carolina | HB 4117 | Makes it a crime to interfere with election officials or election workers holding an election or conducting a canvass so as to prevent, obstruct, impair, or hinder the election or canvass from being fairly held and lawfully conducted | Introduced in 2023 |
| South Dakota | SB-20 | Makes it a crime to directly or indirectly, utter or address any threat or intimidation to an election official or election worker with the intent to improperly influence an election. | Introduced in 2023 |
| Texas | SB-293 | Makes it a crime to use or threatens force, coercion, violence, restraint, damage, harm, or loss against an election official in the performance of their duties and to dox an election official | Introduced in 2023 |
| Texas | HB-3510 | Makes it a crime to intimidate or threaten an election official in the performance of their duties. | Introduced in 2023 |
| Wisconsin | HB-300 | Mandates the state to keep confidential personal information of election officials; expands whistleblower protections to municipal clerks; creates a crime of battery towards election officials | Introduced in 2023 |
| Wisconsin | AB-577 | Makes it a crime to harass an election official; increases penalties for battery ; prohibits public access to personal information. and provides whistleblower protections to officials reporting fraud. | Introduced in 2023 |
| Wyoming | HB-139 | Adds doxxing to other crimes of threats and harassment of election officials. | Introduced in 2023 |
| Wyoming | HB-37 | Makes attacks on election officials aggravated assault. | Introduced in 2024 |
*This list only includes states where election officials are referenced specifically. Many more states such as Idaho and Utah protect elected officials or people working as public officers. These protections apply to some election officials, and may apply to all election workers depending on the state.
Election Officials Are Under Attack
Free and fair elections are the necessary foundation of a healthy democracy, but across the United States, the people responsible for facilitating elections are increasingly under threat:
- Ongoing attacks against local election officials have hindered already underfunded election offices and jeopardized the ability to administer future elections.
- Since 2020, 92% of local election officials have taken steps to increase election security for voters, election workers, and election infrastructure.
- 38% of local election officials report experiencing threats, harassment, or abuse.
- 61% of local election officials who have been threatened say they’ve been threatened in person, and the same number say they’ve been threatened over the phone.
- 34% of local election officials know of one or more local election officials or election workers who have left their job at least in part because of safety fears, increased threats, or intimidation.
- 28% of local election officials say they’re concerned about their family or loved ones being threatened or harassed in future elections.
- 62% of local election officials are worried about political leaders engaging in efforts to interfere in how they or fellow election officials around the country do their jobs.
- 83% of local election officials say their budget needs to grow to meet election administration and security needs over the next five years.
- One in five election officials planned to leave their jobs before 2024.
- In some states, more than half the local election officials have left since 2020. In Arizona, 12 of the state’s 15 county election chiefs have departed. In Pennsylvania, nearly 70 county election directors or assistant directors in at least 40 of the state’s 67 counties have left their jobs.
Many States With Laws Protecting Election Officials Can Still Do More
It is important to note that many states that have laws protecting election officials can do more to provide protections. For example, many states have only protected election officials from doxxing, or the protections are only in the administration of the election and not for acts of retaliation against election officials who simply did their jobs on election day.
Protecting Against “Insider Threats” and Ensuring Elections Are Funded
In addition to protections for election officials, election offices and systems need investment and protection. Due to the added pressures of administering elections, it is important that states increase funding and resources for election officials. Legislation to protect against “insider threats” from election workers such as the critical voting system architecture intentionally taken in Coffee County, Ga. and Mesa County, Colo. is also essential. For example, Colorado and Minnesota (HF 1830) have passed comprehensive protections to codify best practices for election system security, as well as detect, prevent and if needed to penalize actors who tamper with election systems.
See an error or want to share information on state legislation to protect election officials? Please contact Aquene Freechild at afreechild@citizen.org or Jonah Minkoff-Zern at jzern@citizen.org.
Last Updated: May 22, 2024
Stay Updated on Public Citizen
Follow Public Citizen
Support Our Work
Public Citizen Testimony to the Texas House Committee on State Affairs Regarding the Panhandle Wildfires
Download PDF version of comment
To: Chairman Hunter and the Members of the House Committee on State Affairs
CC: Rep. Ana Hernandez, Rep. Rafael Anchía, Rep. Jay Dean, Rep. Charlie Geren, Rep. Ryan Guillen, Rep. Will Metcalf, Rep. Richard Peña Raymond, Rep. Shelby Slawson, Rep. John T. Smithee, Rep. David Spiller, Rep. Senfronia Thompson, Rep. Chris Turner
Via hand delivery and by email.
From: Adrian Shelley, Public Citizen, ashelley@citizen.org, 512-477-1155
Re: Panhandle Wildfires, testimony by Public Citizen
Dear Chairman Hunter and Members of the Committee:
Public Citizen appreciates the opportunity to offer this testify on the findings and recommendations of the Investigative Committee on the Panhandle Wildfires. We must first acknowledge the loss of life that occurred because of these fires. Three people were killed, 15,000 cattle were lost, more than a hundred homes and a million acres of land were burned. This is a tragedy that should never be repeated.
We published a blog on the report in May,1 followed by an op-ed in July.2 Much of this testimony is drawn from those sources. Our recommendations are:
- Acknowledge the role of climate change in the increase in wildfire risk and severity.
- Empower and fund the Public Utility Commission to address power lines and poles as a cause of wildfires.
- Fund the Railroad Commission and task it with remediating orphaned and abandoned well sites. Hold owners and operators liable for wildfires caused by their well sites.
- Fund other proven mitigation strategies.
Texas must acknowledge the role of climate change in making wildfires more common and more severe.
In our op-ed, we were critical of the investigative committee report for ignoring an important cause of wildfires in Texas—climate change. Several causes of wildfires were listed: abundant fuel and a lack of fire breaks, decaying utility poles, and irresponsible oil and gas operators. The report also cites unusual weather conditions of high temperatures, low humidity, and severe wind.
The report suggested that the fires occurring outside of Texas’ normal wildfire season might be a cause of the state being unprepared to combat these fires. The Texas A&M Forest Service (TAMFS) is responsible for predicting wildfires. TAMFS Director Al Davis called the February wildfires “a new phenomenon.” For people living in the heart of Texas wildfire country, exceptionally hot, dry weather is not new. It is becoming the norm.
According to the longtime chief of the Texas Department of Emergency Management, Nim Kidd, the federal government did not take the fire risk in February seriously. This may be true, and failures of state and federal predictions should be considered as a cause of the failed response.
However, at least federal agencies are not afraid to call out climate change as a cause of wildfires. The National Oceanographic and Atmospheric Association directly attributed the conditions that led to the February fires to climate change, “Research has shown that climate change is likely causing the fire season to start earlier and extend longer.”3
If Texas were to acknowledge the role climate change plays in extending wildfire season, the state might not be caught flat footed when fires occur outside of their “normal” season. We urge this committee to take the first step by acknowledging the role that climate change plays in making fires more common, more severe, and more likely to occur throughout the year.
The Public Utility Commission needs more authority to address power lines and poles as a cause of wildfires.
Many of February’s fires were caused by downed power lines and decaying poles. The Grape Vine Creek Fire, the Windy Deuce Fire, and the Reamer Creek Fire were all caused by failing power poles. According to the report, the record-breaking Smokehouse Creek Fire was caused by two companies. A tree wore down power lines on a decayed pole owned by Xcel Energy. A service company—Osmose Utility Service—had identified the pole as needing replacement, but nothing was done. Both Xcel and Osmose were sued for their role in this fire.
Local utility companies are responsible for maintaining poles and lines. The report tasked the Public Utility Commission of Texas with studying and reporting on its procedures to ensure that poles are inspected, restored, and replaced as needed. A study would be useful, but this legislature could do more to empower the PUC to directly address this problem.
State law does not empower the PUC to conduct local inspections of power lines. Although local authorities have the primary role to play in maintaining local lines, the legislature should seriously consider whether giving the PU more authority—and a budget—to conduct local inspections might help address the failure of local entities.
The Railroad Commission is “grossly deficient” in oversight of oil and gas wells, which contributed to many fires.
Many decayed poles and failing wires were traced back to oil and gas wells. Thousands of wells in the panhandle produce only small amounts of hydrocarbons. Irresponsible operators often neglect these marginal or “stripper” wells, which end up orphaned or abandoned. Many have electrical equipment—breaker boxes, wiring, and poles—failing even as power flows through them.
The Texas Railroad Commission is the agency tasked with regulating oil and gas operations in the state. A commission executive testified before the select committee that he was “unaware” that oil and gas operations were causing wildfires across the Panhandle.
The committee recommended that the Railroad Commission “revisit” its system for prioritizing which orphaned wells to address first.
This won’t go far enough. As the report plainly stated, the Railroad Commission is “grossly deficient” in its oversight of wells. The Railroad Commission hardly keeps pace with the rate of newly orphaned wells. It allows active operators to delay well plugging practically indefinitely. In Texas, more than 16,000 inactive wells have been abandoned for twenty years or more.
In order to do its part to address a significant cause of wildfires, the Railroad Commission must address more orphaned and abandoned wells more quickly. In this biennium, it has help from the federal government. The Railroad Commission was able to double its annual well plugging target from 1,000 to 2,000 with more than $60 million per year in federal funding from the Infrastructure Investment and Jobs Act. The table below includes the well plugging allocation each fiscal year, the state contribution from
Account 5155, and the federal allocation, which for 2024-2025 includes funding form the Bipartisan Infrastructure Act4 and the Infrastructure Investment and Jobs Act.
Well Plugging in the Railroad Commission’s Budget5
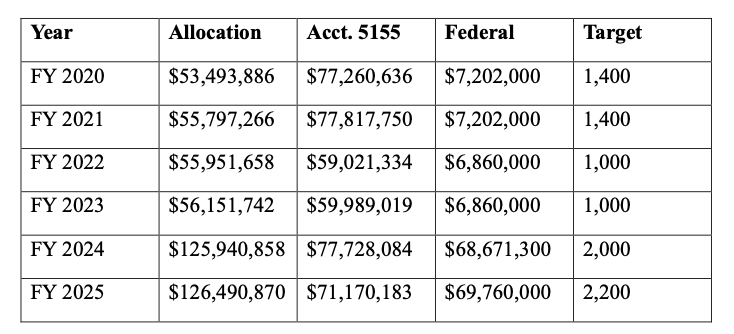
This federal funding is not likely to recur in the next biennium. The legislature should consider whether a larger well plugging budget is necessary to accomplish the Select Committee’s recommendation that the Railroad Commission do more to address orphaned and abandoned wells as a cause of wildfires.
The legislature should also consider whether owners of marginal wells should be held liable for wildfires caused by electrical failures at sites that they own.
The legislature should fund proven mitigation strategies.
There are proven strategies to lessen the impact of wildfires, including suppression lines, fire breaks, green strips, safety zones for firefighters, sprinklers, and training programs. All of these strategies have something in common: they cost money.
Fire prevention and mitigation is woefully underfunded in Texas. Nowhere is it more apparent than in our volunteer fire departments (VFDs). In 2002, the Legislature created a funding program for rural VFDs. But the $23 million allocated last year simply wasn’t enough. The select committee report prioritizes funding VFDs and vesting authority to fight fires with them and their allies in local government.
1 See https://www.citizen.org/news/what-texas-will-and-wont-say-our-look-at-the-panhandle-fires- investigative-report/,.
2 White, Kaiba, “Texas can’t treat climate change like the elephant in the room anymore | Opinion” Austin American-Statesman (8 July 2024) available at https://www.statesman.com/story/opinion/columns/guest/2024/07/08/texas-cant-treat-climate- change-like-the-elephant-in-the-room-any-more/74290950007/.
3 See https://www.nesdis.noaa.gov/news/fires-rage-across-texas-panhandle.
4 https://www.rrc.texas.gov/news/010323-federal-well-plugging-data-visualization/
5 General Appropriations Act 2024-2025, p. VI-52 (pdf p. 708), Railroad Commission Strategy C.2.1, p. VI_56, https://www.lbb.texas.gov/Documents/GAA/General_Appropriations_Act_2024_2025.pdf; General Appropriations Act 2022-2023, p. VI-49 (pdf p. 705), Railroad Commission Strategy C.2.1, https://www.lbb.texas.gov/Documents/GAA/General_Appropriations_Act_2022_2023.pdf; General Appropriations Act 2020-2021, p. VI-48 (pdf p. 702), Railroad Commission Strategy C.2.1, https://www.lbb.state.tx.us/Documents/GAA/General_Appropriations_Act_2020_2021.pdf.
You might be interested in
Stay Updated on Public Citizen
Follow Public Citizen
Support Our Work
Key Facts to Know Before Novo Nordisk’s CEO Appears at the Senate HELP Committee
Download PDF version of comment
On September 24, 2024, Novo Nordisk CEO Lars Jørgensen will testify at the Senate Committee on Health, Education, Labor, and Pensions (HELP) hearing on the high prices of the diabetes and obesity drugs Ozempic and Wegovy.
Here are key facts to know before the hearing:
1. Novo Nordisk charges Americans up to 15 times more than it charges other wealthy countries for Ozempic and Wegovy.
- Novo Nordisk’s pricing isn’t justified by research and development costs. Since Ozempic’s launch in 2018, Novo Nordisk has spent over $44 billion enriching its shareholders through stock buybacks and dividends—over twice as much as it spent on R&D across its entire portfolio.
- Nor is it justified by production costs. Generic Ozempic and Wegovy could be sold profitably for around $5 and $13 per month, respectively. Novo Nordisk prices them over 100 times higher for Americans. Generics firms have indicated they would sell generics for less than $100 per month.
- Novo Nordisk has amassed nearly $50 billion from Ozempic and Wegovy since their launch.
2. Novo Nordisk’s exorbitant pricing in the U.S. has imposed widespread cost barriers on patients and financial burden on public programs.
- The annual cost to the healthcare system for covering Wegovy for half of the eligible population ($411 billion) would exceed the expenditure on all retail prescription drugs in 2022 ($406 billion). Without substantial price reductions, Wegovy could cost the U.S. health care system $1 trillion by 2031, potentially approaching $2 trillion with greater uptake.
- According to a KFF poll, 1 in 8 adults have used GLP-1s (such as Ozempic and Wegovy), with over half saying that it was difficult to afford their costs.
- Between 2020 and 2021, Medicare’s spending on Ozempic increased by more than a billion dollars, and between 2021 and 2022, spending increased by an astonishing $2 billion.
- Officials in North Carolina and West Virginia have raised alarm over how these drugs could cost state health plans covering public employees over $150 million a year, which could increase the cost of premiums in some cases up to 200%.
3. To avert the ruinous financial consequences of Novo Nordisk’s greed, the Biden administration can use its authority under 28 U.S.C. § 1498 to allow generic production of these drugs, resulting in lower costs for patients and federal health programs.
- After unsuccessful efforts to negotiate lower prices for its State Health Plan with GLP-1 manufacturers, North Carolina’s Treasurer requested that HHS initiate efforts to negotiate voluntary licenses between Novo Nordisk and generic drug producers for supply to federal, state, and local government payers.
- If Novo Nordisk refuses to voluntarily license their products, the federal government can use its authority under 28 U.S.C. § 1498 to authorize generic competitors to these price gouged drugs, as described in Public Citizen’s petition to HHS, saving Medicare $14 billion in the first two years alone, even under conservative assumptions of uptake among patients.
Stay Updated on Public Citizen
Follow Public Citizen
Support Our Work
Estimate of Savings from Generic Competitors to Ozempic and Wegovy
Download PDF version of comment
1) Medicare’s savings from generic competition to Ozempic.
Using estimates of net spending for Ozempic in 2022 by Medicare Part D, Public Citizen projects that Medicare would save $1.3 billion in the first year of generic competition and $1.6 billion in the second year of competition to Ozempic. As described in more detail in the Appendix, we estimated savings if the generics cost $100 per month and accounted for increased uptake of generics by patients over time.
| Net Spending on Ozempic in 2022 | Net Spending if Generics were Available | Savings | |
| Year 1 | $2,452,925,141.00
|
$1,147,406,466.61
|
$1,305,518,674.39
|
| Year 2 | $2,452,925,141.00
|
$819,545,498.46
|
$1,633,379,642.54
|
| Total | $4,905,850,282.00
|
$1,966,951,965.07
|
$2,938,898,316.93
|
2) Medicare’s savings from generic competition to Wegovy among enrollees likely eligible due to the new cardiovascular indication.
Federal law prohibits covering drugs solely for weight loss indications. However, as a result of the FDA approving Wegovy to reduce the risk of death, stroke, or heart attack in patients with obesity and excess weight, this year Medicare announced it would allow Part D plans to cover Wegovy for patients with elevated Body Mass Index (BMI) and established cardiovascular disease, regardless of whether the patient has diabetes. Using estimates of the number of Medicare enrollees who likely become eligible for Wegovy under this new cardiovascular indication (3.6 million), Public Citizen estimates Medicare would save between approximately $5 and $20 billion in the first year of generic competition to Wegovy. The range results from the lack of certainty on how many of the 3.6 million enrollees eligible for Wegovy will actually use the medication, so we constructed a lower and upper bound estimate of savings depending on 25% uptake among eligible enrollees vs. 100% uptake among these patients. Similarly, Public Citizen estimates that Medicare would save between approximately $6 billion and $25 billion from the second year of generic competition to Wegovy.
Even under the more conservative set of assumptions, where 25% of Medicare’s eligible enrollees use Wegovy, the program would save over $11 billion in the first two years of generic competition due to Wegovy’s sky high price currently. If even half of Medicare enrollees now eligible for Wegovy use the drug, Medicare would save over $20 billion from the first two years of generic competition on the drug. Finally, under more generous estimates of uptake of Wegovy among eligible enrollees (100% uptake), we estimate over $45 billion in savings from the first two years of generic competition.
| Savings if 25% of Eligible Medicare Enrollees Use Wegovy | Savings if 50% of Eligible Medicare Enrollees Use Wegovy | Savings if 100% of Eligible Medicare Enrollees Use Wegovy | |
| Year 1 | $5,061,409,200.00 | $10,122,818,400.00 | $20,245,636,800.00 |
| Year 2 | $6,332,504,400.00 | $12,665,008,800.00
|
$25,330,017,600.00 |
| Total | $11,393,913,600.00
|
$22,787,827,200.00
|
$45,575,654,400.00 |
3) Savings to Medicare if the program covers Wegovy for enrollees with an obesity diagnosis.
Bicameral, bipartisan legislation has been proposed to end the Medicare coverage exclusion for drugs used for the treatment of obesity or for weight loss management for individuals with excess weight and who have one or more related comorbidities.[1] We calculate that if Medicare covered Wegovy just for enrollees with an obesity diagnosis, the program would save between approximately $15.7 and $62.7 billion in the first year of generic competition to Wegovy. In the second year of generic competition to Wegovy, Medicare would save between approximately $19.6 and $78.4 billion.
Under the more conservative set of assumptions, we anticipate that if Medicare covers Wegovy for just 25% of enrollees with an obesity diagnosis, generic competition will save the program over $35 billion in just two years. If even half of Medicare enrollees with an obesity diagnosis used the drug, we anticipate that generic competition would save the program over $70 billion over two years. If all Medicare beneficiaries with an obesity diagnosis took the drug, generic competition would save the program over $140 billion in two years.
| Savings if 25% of Medicare Beneficiaries w/ Obesity Use Wegovy | Savings if 50% of Medicare Beneficiaries w/ Obesity Use Wegovy | Savings if 100% of Medicare Beneficiaries w/ Obesity Use Wegovy | |
| Year 1 | $15,664,511,530.80 | $31,329,023,061.60 | $62,658,046,123.20 |
| Year 2 | $19,598,413,065.01 | $39,196,826,130.02
|
$78,393,652,260.05
|
| Total | $35,262,924,595.81
|
$70,525,849,191.63
|
$141,051,698,383.25 |
Appendix: Methodology for Calculating Savings from Generic Competition on Ozempic and Wegovy
1) Medicare’s savings from generic competition to Ozempic.
To estimate savings to Medicare we used the following data sources and assumptions. According to Medicare’s gross spending data for the latest year available (FY 2022), Medicare spent $4,628,160,643.40 on Ozempic.[2] We used the Government Accountability Office’s estimate of rebates as a proportion of gross expenditure for Part D endocrine metabolic agents (47%),[3] which includes antidiabetic medications, to estimate net spending on Ozempic in 2022. We estimate net spending for Ozempic in 2022 to be approximately $2,452,925,141.00.
Next, we calculated how much Medicare would save if patients began using generics rather than the expensive branded medication. First, we estimated continued spending on the branded drug, Ozempic, given academic literature on generic uptake in the first and second year of competition. We prefer this approach to account for the fact that generic uptake increases over time. Experts have found that in the first year of generic competition for a drug, there is 66.1% uptake of generics; in the second year, uptake of generics increases to 82.7%.[4] For the first year of competition, we estimated that Medicare would continue to spend $831,541,622.80 and $424,356,049.39 on the branded medication, Ozempic, in the first and second year of generic competition, respectively.
Then, we calculated net expenditure on generics that would occur as a function of displacing Ozempic’s market share with an estimated price reduction compared to the brand price. The brand price for Ozempic is $968.52 per month.[5] Although inexact, we applied the Government Accountability Office’s rebate estimate for gross spending on endocrine metabolic agents in Medicare Part D to estimate a net price of $513.32 per month per enrollee. If generics can enter the market at $100 a month, the price ratio of generics compared to Ozempic would be 0.194811925. Thus, using this price ratio and expected uptake of generics in the first and second year of competition, we estimated that spending on generics would be $315,864,843.81 and $395,189,449.07 in the first and second year of competition, respectively.
We totaled the continued spending on the branded medication and generics to calculate how much Medicare would spend if generics to Ozempic became available. In the first year, we estimate that Medicare would spend $1,147,406,466.61 on Ozempic and its generics. In the second year, Medicare would spend $819,545,498.46. Compare this to how much Medicare is expected to spend on Ozempic without generics each year: $2,452,925,141.00. Thus, as a consequence of generic competition, Medicare is expected to save 1,305,518,674.39 in the first year and $1,633,379,642.54 in the second year of generic competition, for a total savings of nearly $3 billion in the first two years of generic competition to Ozempic ($2,938,898,316.93).
Caveats to this analysis include that we cannot account for increased uptake of generics to Ozempic because of their lower price. Second, we do not have precise information on the rebates provided by the branded manufacturer to Medicare Part D; however, we believe the savings here are underestimates because spending on Ozempic has dramatically increased recently. Between 2020 and 2021, Medicare’s spending on Ozempic increased by more than a billion dollars, and between 2021 and 2022, spending increased by an $2 billion. Thus, we likely significantly underestimate how much Medicare will spend on Ozempic in subsequent years since we depend on data from 2022, which also limits our savings projections.
2) Medicare’s savings from generic competition to Wegovy among enrollees likely eligible due to the new cardiovascular indication.
There is no historic data on Medicare’s spending on Wegovy due to statutory exclusions for coverage of drugs solely for weight loss. However, with Medicare’s announcement that Part D plans may begin to cover the drug for patients with elevated BMI and established cardiovascular disease, we relied on estimates in the academic literature. Experts in the Annals of Internal Medicine estimated that 3.6 million enrollees were highly likely to be eligible for Wegovy due to the new cardiovascular indication.[6] Under more liberal definitions of established cardiovascular disease, the authors predicted over 15 million enrollees could become eligible for Wegovy.[7] For the purposes of this analysis, we chose the more conservative estimate of 3.6 million enrollees becoming eligible for Wegovy under Medicare’s recent announcement.
We projected estimated net spending for Wegovy under Medicare if 25% of the enrollees highly likely to be eligible used the medication. Relying on prior estimates of the net price for Wegovy ($809 per month) from the Senate Committee on Health, Education, Labor and Pensions,[8] we calculated an annual net expenditure of $8,737,200,000.00 for these patients.
We used the methodology described above to calculate anticipated savings from generic competition in the first and second year of generics availability. We assumed generic uptake would be 66.1% and 82.7% in the first and second year of competition, respectively,[9] and we calculated the generic-to-brand price ratio based on the net price of $809 for Wegovy and $100 for generics (0.123609394). In the first year of generic competition, we anticipated that Medicare would continue to spend $2,961,910,800.00 on Wegovy. With the availability of generics, we project that Medicare would spend $713,880,000.00 on generics for enrollees. So, under the scenario with generics displacing Wegovy’s market share at a lower price, we expect total spending to be $3,675,790,800.00 as opposed to $8,737,200,000.00 (the net spending projected for Medicare if just 25% of likely eligible enrollees used Wegovy). That equates to savings of over $5 billion under this conservative scenario where only 1 in 4 eligible enrollees use the drug.
In the second year of generic competition, we estimate that Medicare would spend $1,511,535,600.00 on the brand medication, Wegovy, and $893,160,000.00 on generics. With a greater share of Wegovy’s market displaced in the second year and the lower price of generics, we anticipate total spending to be $2,404,695,600.00 under the conservative projection that only 25% of eligible enrollees use the drug. Compared to the estimated net expenditure on Wegovy without any generic competition ($8,737,200,000.00), we estimate over $6 billion in savings from the second year of generic competition. Thus, under a conservative scenario where only 1 in 4 Medicare enrollees eligible for Wegovy under the cardiovascular indication use the drug, we estimate $11,393,913,600.00 in savings from two years of generic competition.
If half of all enrollees highly likely to be eligible for Wegovy use the drug, we would anticipate net expenditures and savings to double. Without generic competition to Wegovy, net expenditure each year would be $17,474,400,000.00, and savings across two years of generic competition would be $22,787,827,200.00. If all enrollees who are highly likely to be eligible for Wegovy under the cardiovascular indication use the drug, we anticipate net spending per year to be $34,948,800,000.00 and savings after two years of generic competition to be $45,575,654,400.00.
As we stated above, there are several caveats to this analysis. First, we cannot account for increased usage of generics to Wegovy as a result of their lower price. Second, we do not have precise information on the rebates provided by the branded manufacturer to Medicare Part D for Wegovy; however, we believe our analysis provides an accurate range of potential spending for Medicare because we chose the stricter definition of cardiovascular disease in the literature and showed that Medicare stands to save over $11 billion in two years of generic competition in the conservative scenario where only 1 in 4 enrollees under this strict definition of cardiovascular disease use Wegovy. Thus, even if the final rebate amount is modified, we anticipate that if Medicare were to cover Wegovy for the weight loss indication, savings would be at least, and likely exceed, $11 billion across two years of generic competition because level of uptake and the definition of cardiovascular disease would significantly affect potential net expenditures and savings. For example, if more liberal definitions of cardiovascular disease are used, where over 15 million enrollees may be eligible for Wegovy instead of 3.6 million enrollees, our analysis may grossly underestimate potential spending on Wegovy and savings from generic competition.
3) Savings to Medicare if the program covers Wegovy for enrollees with an obesity diagnosis.
In light of bicameral, bipartisan legislation that would end the Medicare coverage exclusion for drugs used for the treatment of obesity or for weight loss management for individuals with excess weight and who have one or more related comorbidities,[10] we provide an estimate of potential savings to Medicare from generic competition to Wegovy if this exclusion were ended.
According to an article from the New England Journal of Medicine, experts found that 21% of Medicare Part D enrollees in 2020 had a diagnosis of obesity (9,956,755 enrollees).[11] Extrapolating to 2024, where Medicare Part D’s enrollment has increased by 11.9% since 2020, we estimate that 11,141,609 enrollees likely have an obesity diagnosis.[12] Using the net price for Wegovy for Medicare discussed above from the Senate HELP Committee’s report,[13] we estimate the annual net expenditure if Medicare ended the exclusion for anti-obesity medications and just 25% of those with an obesity diagnosis used Wegovy. We note this is likely an underestimate of the number of enrollees who would use Wegovy, not only because of the conservative estimate of uptake, but because this does not consider usage by patients who are overweight and have one or more weight-related comorbidity. We project, conservatively, that Medicare would spend on a net basis at least $27,040,684,666.82 covering the weight loss indication.
Using the methodology discussed above, we projected savings to Medicare with increasing generic uptake and displacement of the branded drug, Wegovy over time using the generic-to-brand price ratio. Again, we assumed generic uptake would be 66.1% and 82.7% in the first and second year of competition, respectively,[14] and we calculated the generic-to-brand price ratio based on the net price of $809 for Wegovy and $100 for generics (0.123609394). In the first year of generic competition, we calculated continued spending on Wegovy would be $9,166,792,102.05 while spending on generics would be $2,209,381,033.96. In total, we expect spending with generics available to be $11,376,173,136.01, representing $15,664,511,530.80 in savings in just the first year compared to what Medicare would expend with no generic alternatives to Wegovy ($27,040,684,666.82).
In the second year of competition, we would expect spending on Wegovy to drop to $4,678,038,447.36 and spending on generics to be $2,764,233,154.44. Thus, with generics available, we would expect a total spend of $7,442,271,601.80 as opposed to $27,040,684,666.82 without generic alternatives. That represents $19,598,413,065.01 in savings during the second year of competition under the conservative scenario in which 1 in 4 Medicare enrollees with obesity used this anti-obesity medication. Across two years of generic competition to Wegovy, we anticipate at least $35,262,924,595.81 in savings if the Medicare coverage exclusion for anti-obesity treatment is ended.
If 50% of Medicare enrollees with an obesity diagnosis took Wegovy upon ending the coverage exclusion, we anticipate that annual net expenditure would double to $54,081,369,333.63, and savings from two years of generic competition would be $70,525,849,191.63. If all Medicare enrollees with an obesity diagnosis took Wegovy, we would expect annual expenditures to be $108,162,738,667.26 and savings from two years of generic competition to approximate $141,051,698,383.25.
As described above, we cannot account for increased uptake of this anti-obesity medication due to lower costs from generic competition. Differences between the estimated net price here and the actual net price paid by Medicare will also impact this analysis. But we believe we’ve accurately projected the range of spending and savings possible by using conservative assumptions on levels of uptake that greatly affect possible net expenditures and savings. That is, if only 1 in 4 Medicare beneficiaries with an obesity diagnosis take Wegovy, we still project savings over $35 billion across two years of generic competition. Thus, even if the final rebate amount is modified, we anticipate that if Medicare were to cover Wegovy for the weight loss indication, savings would be at least, and likely exceed, $35 billion across two years of generic competition.
[1] Treat and Reduce Obesity Act of 2023, S.2407, 118th Cong. (2023); H.R.4818, 108th Cong. (2023) https://www.congress.gov/bill/118th-congress/senate-bill/2407.
[2] Ctrs. Medicare & Medicaid Servs., Medicare Part D Spending by Drug, Data.CMS.Gov, https://data.cms.gov/summary-statistics-on-use-and-payments/medicare-medicaid-spending-bydrug/medicare-part-d-spending-by-drug (last visited Sept. 16, 2024).
[3] U.S. Gov’t Accountability Off., Medicare Part D: CMS Should Monitor Effects of Rebates on Plan Formularies and Beneficiary Spending, at 19 (Sept. 2023), https://www.gao.gov/assets/gao-23-105270.pdf.
[4] Benjamin N. Rome, ChangWon C. Lee, Joshua J. Gagne, & Aaron S. Kesselheim, Factors Associated With Generic Drug Uptake in the United States, 2012 to 2017, 24 Value Health 804 (2021).
[5] Novo Nordisk, Find out the cost for Ozempic, NovoCare, https://www.novocare.com/diabetes/products/ozempic/explaining-list-price.html (last visited Sept. 16, 2024).
[6] Alexander Chaitoff, Liam Bendicksen, William B. Feldman, Alexander R. Zheutlin, & Hussain S. Lalani, Estimating New Eligibility and Maximum Costs of Expanded Medicare coverage of Semaglutide for Cardiovascular Prevention, Annals of Internal Medicine (Aug. 27, 2024), https://www.acpjournals.org/doi/10.7326/ANNALS-24-00308
[7] Id.
[8] Majority Staff of the Senate HELP Committee, Breaking Point: How Weight Loss Drugs Could Bankrupt American Health Care 6-7 (May 15, 2024).
[9] Benjamin N. Rome, ChangWon C. Lee, Joshua J. Gagne, & Aaron S. Kesselheim, Factors Associated With Generic Drug Uptake in the United States, 2012 to 2017, 24 Value Health 804 (2021).
[10] Treat and Reduce Obesity Act of 2023, S.2407, 118th Cong. (2023); H.R.4818, 108th Cong. (2023) https://www.congress.gov/bill/118th-congress/senate-bill/2407.
[11] Khrysta Baig, Stacie B. Dusetzina, David D. Kim, & Ashley A. Leech, Medicare Part D Coverage of Antiobesity Medications— Challenges and Uncertainty Ahead, 388 New England J. Med. Perspective 961 (2023).
[12] Juliette Cubanski & Anthony Damico, Key Facts About Medicare Part D Enrollment, Premiums, and Cost Sharing in 2024, KFF (July 2, 2024), https://www.kff.org/medicare/issue-brief/key-facts-about-medicare-part-d-enrollment-premiums-and-cost-sharing-in-2024/.
[13] Majority Staff of the Senate HELP Committee, Breaking Point: How Weight Loss Drugs Could Bankrupt American Health Care 6 (May 15, 2024).
[14] Benjamin N. Rome, ChangWon C. Lee, Joshua J. Gagne, & Aaron S. Kesselheim, Factors Associated With Generic Drug Uptake in the United States, 2012 to 2017, 24 Value Health 804 (2021).
Stay Updated on Public Citizen
Follow Public Citizen
Support Our Work
Public Citizen Testimony to the Texas Senate Committee on Natural Resources & Economic Development Regarding Cement Production Plants
Download PDF version of comment
To: Chairman Brian Birdwell and the Members of the Senate Committee on Natural Resources & Economic Development
CC: Sen. Judith Zaffirini, Sen. Carol Alvarado, Sen. César Blanco, Sen. Kelly Hancock, Sen. Bryan Hughes, Sen. Lois W. Kolkhorst, Sen. Borris L. Miles, Sen. Kevin Sparks
Via hand delivery and by email.
From: Adrian Shelley, Public Citizen, ashelley@citizen.org, 512-477-1155
Re: Cement Production Plants, Public Citizen testimony
Dear Chairman Birdwell and Members of the Committee:
Public Citizen appreciates the opportunity to offer this testimony. There are significant community concerns about the BM Dorchester LLC Dorchester Cement Production Plant in Grayson County. We agree with these concerns and are, as always, sympathetic to the impacted community. We also recognize that the problems identified in this proposal and within this industry are not unique to either—they are common across Texas Commission on Environmental Quality (TCEQ).
We urge this committee to view this issue in the larger context of a state environmental permitting system that does not protect public health or the environment. A solution that addresses TCEQ’s systemic failures will help Grayson County and countless other communities across Texas.
There are significant community concerns with the Dorchester Cement Production Plant proposal in Grayson County.
The concerns raised by the community impacted by the Dorchester Cement Production Plant are well articulated in EPA’s comments to TCEQ on the permit application:1
EPA is aware of numerous community concerns regarding the proposed project. Many of these concerns focus on the anticipated impact of plant operations on the surrounding community, such as air quality degradation, increased traffic, and the potential for nuisance conditions. The roads in this area are narrow and unpaved and the proposed source’s fenceline is adjacent to a place of worship and residential areas. EPA is concerned by the potential for heavy vehicle traffic, both on and off-property, to generate particulate matter emissions that could migrate beyond the property line. The facility may also be a source of noise and light pollution. When present, these potential nuisance conditions could impact residents’ quality of life and may interfere with the normal use and enjoyment of their property, nearby parks, schools, and other outdoor public spaces. As TCEQ is aware, 30 Texas Administrative Code § 101.4 prohibits nuisance conditions, and therefore TCEQ is able to address community concerns as they arise.
The nearby town of Dorchester is home to around 100 people. There is a neighborhood platted nearby for thousands of new homes. There is a church near the proposed facility that is more than 100 years old. There is a nearby airport. Concerns have also been raised about a semiconductor manufacturer located some six miles away. This manufacturer is concerned about air pollution and seismic effects from blasting. It is our understanding that the permit application has decided not to proceed with the blasting operation at the facility.
There are cement production plants throughout Texas.
This map by Air Alliance Houston shows each of the twelve cement kilns in Texas.2 There are three south of Dallas, five between San Antonio and Austin, and one each in Houston, Waco, Odessa, and Maryneal.

Texas has a difficult history with cement production plants that continues to this day.
The history of cement plant permitting in Texas shows that these facilities ca3n be significant health hazards for their neighbors. Rita Beving, our organizer in Dallas, participated in an extended fight against the TXI facility between 1995 and 2000.3 Her account of this issue follows:
“Midlothian, TX, a half hour southwest of Dallas, is considered the cement capital of Texas. In the 90s, it was home to three cement kilns which burned hazardous waste to make cement – TXI, Holcim, and North Texas Cement.
“During this time, if the kiln burned hazardous waste, a company was paid to take hazardous waste from companies such as auto body shops or dry cleaners. Hazardous waste was imported from all over the country and as far as Puerto Rico. At one time, TXI even considered burning napalm for the military.
“Because they were considered “recyclers,” these plants were not required to have scrubbers on their stacks unlike government incinerators.
“They were not required to deposit the ashes from the process into lined, manifested landfills like other more regulated entities. Instead, ashes were often dumped on site. In TXI’s case, ashes blew into Cottonwood Creek and flowed into Joe Poole Lake.
“The stacks on these plants are very tall. Without scrubbers, they produced a toxic soup of pollutants including NOx, VOCs, SO2, sulphuric acid, PM10 and PM 2.5. Other pollutants included beryllium, cadmium, chromium, arsenic, lead, and mercury. Because some of these plants burned tires, emitted pollutants could even include chlorine gas and dioxins— one of the most carcinogenic pollutants known.
“Modeling commissioned by opponents of TXI’s permit proved that pollution traveled at least thirteen miles from the plant. The contested case hearing against TXI’s permit eventually included thirty-five litigants.
“The Texas Department of State Health Services confirmed the existence of a cluster of birth defects including Down Syndrome in the area around Midlothian.4 DSHS also identified high rates of breast cancer. We learned of horses growing extra muscles, ostrich eggs that would never hatch, dogs born with bent legs. There were adolescent children and adults with rare forms of brain cancer. We found lots of autism. We had mothers testifying that when they were not in Midlothian on vacation or outside the city, that their kids’ asthma improved.
“More than 30 doctors in Cedar Hill, Dallas, DeSoto, and elsewhere opposed the TXI permit and wrote letters about the harm to the community they had already seen in their practices.
“The case was one of the longest contested case hearings in Texas history. After years the protestants lost in the Texas Supreme Court where we lost. The permit’s opponents spent more than $250,000 in the late 90s. Even the cities of Duncanville and DeSoto participated in the TXI permit fight, each contributing $25,000.
“This case was of keen interest to the Portland Cement Association, as getting selective catalytic reduction (SCR) or selective non-catalytic reduction (SNCR) scrubbers would set a precedent for the nation as far as future kiln regulation from the EPA.
“Of the three facilities still located south of Dallas, Martin Marietta owns TXI,5 LaFarge owns Holcim,6 and Ash Grove owns the North Texas Cement Company.7
“These plants do not burn hazardous waste today, but they still emit NOx, VOCs, SO2, PM10 and PM2.5, affecting the health of local residents. Most plants today burn methane gas. Ash Grove modernized its operation in 2016 to9 still allow the burning of tires, which would produce chlorine and dioxins.8 Ash Grove added selective non-catalytic reduction (SNCR) which “reduced NOx by 60% since 1996.”9
“Holcim now burns both tires (15% of fuel base) and PLASTICs (8% of fuel base) according to their 2022 fact sheet.10
“TXI still burns tires and announced in 2022 that it would increase its fuel supply by adding shredded tires.11 Again, this raises concerns about chlorine and dioxin pollution.”
Permitting concerns in Texas are not limited to the cement production industry.
We must acknowledge that concerns about permitting in Texas are not limited to the cement production industry. Over four decades of operation in Texas, Public Citizen has seen community concerns from industries as diverse as electric generation (including coal, and gas), oil refining, petrochemical manufacture, fertilizer manufacture, metal recycling, cement production, and aggregate production operations including quarries, asphalt plants, rock crushers, and concrete batch plants.
To understand why this problem is with TCEQ itself—not just the cement production industry—it is useful to quote from Lt. Gov. Dan Patrick’s April 16, 2024 letter to TCEQ Chairman Niermann about the Dorchester Cement Production Plant proposal.12 The first paragraph of Lt. Gov. Patrick’s letter concludes:
Business leaders, clergy, elected officials, community leaders, and an overwhelming majority of the public have all voiced their objections to the Texas Commission on Environmental Quality (TCEQ) granting a permit to Black Mountain Cement for this project.
This sort of universal opposition to a proposed permit is not common. Most permitted facilities are good actors and good neighbors. But when opposition is this widespread, TCEQ is still unmoved.
Lt. Gov. Patrick’s own pursuit of this issue, including through a site visit and a town hall, led him to conclude that:
[I]t is clear to me from my visit that there is more to consider, and to move forward would cause the entire community great damage now and for the foreseeable future.
However, as Lt. Gov. Patrick correctly notes, TCEQ leadership is unable to grant the remedy he requests—that the permit not be granted. This is because, in his words:
I appreciate that TCEQ has a difficult job. You have a formula, and you follow it.
Emphasis added. This final point gets to the heart of the issue. The Texas Commission on Environmental Quality has long held the position that it does not have the authority to deny a permit that is otherwise administratively and technically complete.
We believe this is an incorrect interpretation of the Texas Clean Air Act. We raised this issue during TCEQ’s Sunset Review last session.13 Nevertheless, this is how TCEQ operates. This interpretation of its authority makes TCEQ’s public participation process a futile exercise.14 Why should anyone participate in a permit process when the outcome is foreordained? It is true that sometimes facilities don’t get built. The applicant might itself decide not to site in a hostile community. In one rare case, the TCEQ was forced to deny a permit after litigation uncovered an error in the relevant statutes.15
But what doesn’t happen is that the TCEQ decides not to grant a permit because a facility just doesn’t belong in a given location. In fact the agency doesn’t believe it even has the authority to do so.
The legislature could act to give the Commission explicit authority to do what seems obvious—deny permits that should not be granted due to considerations of health, safety, or justice.
As Lt. Gov. Patrick said in his letter:
[A]s Lt. Governor, I must look at the bigger picture of what is best for our communities.
This just makes sense. Shouldn’t TCEQ have the same ability?
Conclusion: these issues will reoccur until the larger problem with TCEQ is addressed.
Finally, we want to acknowledge the many community members from across Texas who made a difficult decision not to come to today’s hearing. They felt that it would be useful to this committee to share their own similar challenges with environmental permitting of other types of facilities. Some of those facilities, such as concrete batch plants and rock crushers, are admittedly not cement production plants. But the communities surrounding them face similar issues such as air pollution, truck traffic, and nearby sensitive land uses.
Again, we hope that the members of this committee appreciate that the challenges we are discussing today are not unique to cement production plants. They occur across dozens of industries and thousands of facilities throughout Texas. People across the state have been in the position that the people of Grayson County are in now. Perhaps the people of Grayson County could have learned from their experiences.
But those people were encouraged not to come today. We hope that their opportunity will come sooner rather than later during the 89th legislative session.
1 Letter from Cynthia J. Kaleri Air Permits Section Manager to Ms. Laurie Gharis, Chief Clerk, TCEQ “Re: Clean Air Act (CAA) New Source Review (NSR) Permits for the BM Dorchester LLC, Dorchester Cement Production Plant (Dorchester Plant), located in Grayson County, Texas – RN111368437 – Initial Permit Nos. 167047, PSDTX1602, and GHGPSDTX212” (25 Mar. 2024).
2 See https://www.arcgis.com/home/webmap/viewer.html?webmap=62ceba9778ed44a599bea4e3d50807c 8.
3 See generally, https://www.dallasobserver.com/news/ill-wind-blowing-6402678.
4 See e.g. https://www.atsdr.cdc.gov/sites/midlothian/docs/Midlothian-Updated-PHRP-May-2012.pdf, https://www.nrdc.org/sites/default/files/texas_diseaseclusters.pdf.
5 See https://ir.martinmarietta.com/static-files/8684d9ae-52cc-483d-95b5-7ea933f10024.
6 See https://www.holcim.us/sites/us/files/2022-03/MIDLOTHIAN_Fact_Sheet_March2022_HR.pdf.
7 See https://www.globalcement.com/magazine/articles/981-a-visit-to-ash-grove-s-midlothian- cement-plant.
8 Id.
9 Id.
10 See https://www.holcim.us/sites/us/files/2022-03/MIDLOTHIAN_Fact_Sheet_March2022_HR.pdf.
11 See https://www.martinmarietta.com/about-us/article?id=pkHffX8mxbK1LhcAmoBY.
12 See https://www.ltgov.texas.gov/2024/04/16/lt-gov-dan-patrick-sends-letter-to-texas-commission-on-environmental-quality-tceq-chairman-jon-niermann/.
13 See, e.g., Sec. I.B. of our comments at https://www.citizen.org/article/our-proposal-to-reform-the- tceq/.
14 In our experience, the Contested Case Hearing process can improve the public health and safety protections in a permit, or otherwise improve the relationship between the permit holder and its neighbors. But only a small number of permits ever enter a Contested Case Hearing.
15 See https://spectrumlocalnews.com/tx/south-texas-el-paso/news/2021/06/11/mansfield-neighbors- relieved-after-permit-for-concrete-batch-plant-in-their-neighborhood-is-denied.
Stay Updated on Public Citizen
Follow Public Citizen
Support Our Work
Protest of BlackRock’s Acquisition of Allete
By Tyson Slocum
Download PDF version of comment
Today in Federal Energy Regulatory Commission docket EC24-105, the Private Equity Stakeholder Project joined with Public Citizen in protesting BlackRock’s effort to acquire Allete and its franchised utilities Minnesota Power and Superior Water, Light and Power Company.
BlackRock’s proposed acquisition of ALLETE and its franchised utilities fails the public interest test required under Section 203 of the Federal Power Act[1] because BlackRock’s extensive horizontal control of utility and end-user voting shares subject to blanket authorization threatens competition, rates and regulation. The proposed ALLETE transaction and approved acquisition of GIP change the “facts, policies, and procedures the Commission relied upon in granting” BlackRock’s blanket waivers to control up to 20% of the voting shares of utilities, and as such first requires BlackRock to “inform the Commission within 30 days of any material change in circumstances that would reflect a departure from the facts, policies, and procedures the Commission relied upon in granting the” blanket authorization.[2] The Commission must therefore hold this proceeding in abeyance until the Commission completes its review of how the GIP acquisition and proposed ALLETE transaction materially change the facts of BlackRock’s blanket authorization, as required by the Commission’s BlackRock-GIP order.[3] Absent changes to or revocation of that blanket authorization, the Commission cannot find the proposed transaction to “be consistent with the public interest” as required by law.
Commissioner Mark C. Christie noted in his concurring statement on the Commission’s approval of BlackRock’s acquisition of GIP the “influence that large shareholders, BlackRock or otherwise, can potentially exert across the consumer-serving utility industry should not be underestimated. Such horizontal shareholdings pose the threat of decreased innovation, reduced competition, and ultimately higher prices to consumers, as well as the prospect of chilling investment” in generation . . . You do not need a Ph.D. in economics to see the potential for anticompetitive conduct and outcomes when an investment entity like a huge asset manager seeks to own generation assets that will be — or should be — competitors.”[4] This is most certainly the case with BlackRock’s proposed acquisition of ALLETE.
BlackRock already controls 13.01% of ALLETE voting shares,[5] held through the Commission’s blanket authorization. BlackRock’s control of 13% of ALLETE’s voting shares exposes the active conflict of interest in the Commission’s blanket authorization policy, as BlackRock can use its control of ALLETE’s voting shares to further its financial self-interest ahead of ALLETE’s captive customers. The Commission’s blanket authorization policy fails to remedy BlackRock’s self-interest in voting those shares.
BlackRock also controls 10.81% and 10.41%,[6] respectively, of Cleveland Cliffs and US Steel—two industrial taconite facility customers of ALLETE representing 70% of Minnesota Power’s entire industrial demand.[7] BlackRock’s control of 13% of ALLETE’s voting shares and more than 10% of two of its largest customers raises concerns about horizontal competition and conflict of interest that are ignored in the application. The application is silent on how BlackRock can simultaneously manage its non-controlling ownership of voting shares of utilities that compete with its active, direct holdings—an income prioritization conflict for BlackRock that threatens competition, rates and regulation.
According to BlackRock’s most recent quarterly filing, submitted to the Commission on August 14, 2024,[8] it controls voting shares in excess of 10% of at least 19 different electric utilities operating in FERC-jurisdictional markets: Avista Corp; Black Hills Corp; Chesapeake Utilities; Consolidated Edison; Essential Utilities; Eversource Energy; Exelon Corp; Idacorp; MGE Energy; New Jersey Resources Corp; Northwestern Energy; OGE Energy; Otter Tail Corp; PNM Resources; Portland General Electric; Public Service Enterprise Group; Sempra; UGI Corp; and Unitil Corp. This same disclosure reveals BlackRock controls 18.68% and 10.57%, respectively, of the voting shares of SunRun and FirstSolar; 13.63% of coal supplier Peabody Energy; additional companies that sell services to utilities (Hannon Armstrong 15.4% and Ormat Technologies 13.93%), and entities with MBR affiliates (Stepstone Group, 12.12%).
The proposed ALLETE purchase fundamentally transforms BlackRock’s business model from that of a non-controlling asset manager to acquiring securities that will result in the transfer of control over a public utility, rendering the facts and conditions of the blanket authorization to be null and void. Evidence of BlackRock’s transformation from a passive to active investor includes the fact that GIP’s CEO, Adebayo Ogunlesi, will be promoted to Blackrock’s board of directors and global executive committee, giving him oversight of Blackrock’s overall business (both active and non-controlling investments). This promotion of GIP’s CEO to BlackRock’s board of directors undermines the application’s claim that “the strategic and day-to-day operations and activities of [GIP] will continue to be organized and managed separately from BlackRock.”[9] GIP cannot be “managed separately from BlackRock” when GIP’s current CEO will literally serve on BlackRock’s board and global executive committee. BlackRock’s failure to segregate its non-controlling investment management operations from its active control of ALLETE and other Commission-jurisdictional assets clearly violates the terms of the Commission’s blanket authorization order and is therefore a fatal error of the application.
The application’s failure to remedy how the proposed ALLETE acquisition violates BlackRock’s blanket authorization must result in either the Commission rejecting the application for being inconsistent with the public interest; modifying the terms of the blanket authorization; or requiring divestiture of BlackRock’s non-controlling investment management operations from its active investments. At a minimum, the Commission should require applicants to include in its market power screens all MBR entities where BlackRock controls voting shares of affiliates in excess of 10%, even when that control is subject to the Commission’s blanket authorization.
ALLETE filed petitions to approve the acquisition on July 19th at the Minnesota Public Utilities Commission[10] (MNPUC) and the Public Service Commission of Wisconsin[11] (PSCW). The Citizens Utility Board of Minnesota (CUB) submitted a comment stating that it is “concerned about the inherent conflict of interest that arises between ratepayers wanting to pay as little as possible for safe and reliable electric service and private equity investors wanting to maximize their return on investment.”[12] In addition, Minnesota Attorney General Keith Ellison filed a comment that includes an analysis of whether the transaction is consistent with the public interest. The analysis states: “If the transaction is consummated, ALLETE and Minnesota Power will become subsidiaries of Global and Canada Pension. Global is itself set to be acquired by BlackRock, one of the world’s largest asset managers. All of these entities have numerous other investments in energy and nonenergy industries, presenting opportunities for cross-subsidization and self-dealing that could harm Minnesota Power’s ratepayers and the public interest more broadly.”[13] The comment letter recommends the case be sent to an evidentiary hearing to determine whether the proposed transaction may have negative implications for its ratepayers, Minnesota and the public interest. In addition, the MN Department of Commerce also voiced concerns over the deal saying it “raises significant issues related to affiliated interests”.[14]
The Commission must hold this proceeding in abeyance until it completes its review of how BlackRock’s GIP acquisition and proposed ALLETE transactions materially change the facts of BlackRock’s blanket authorization.
Access a .pdf of the filing here ALLETE protest
[1] 16 U.S.C. § 824b(a)(4).
[2] April 19, 2022 Order Extending Blanket Authorization to Acquire Securities, Docket No. EC16-77-002, at page 12, paragraph M, https://elibrary.ferc.gov/eLibrary/filelist?accession_num=20220419-3080
[3] September 6, 2024 “Order Authorizing Disposition of Jurisdictional Facilities and Acquisition of Securities,” 188 FERC ¶ 61,166, Docket No. EC24-58 at ¶58, https://elibrary.ferc.gov/eLibrary/filelist?accession_num=20240906-3099
[4] Docket No. EC24-58, at ¶2, https://elibrary.ferc.gov/eLibrary/filelist?accession_number=20240715-5129
[5] BlackRock’s most recent quarterly filing, submitted to the Commission August 14, 2024
https://elibrary.ferc.gov/eLibrary/filelist?accession_number=20240814-5165
[6]BlackRock’s most recent quarterly filing, submitted to the Commission August 14, 2024
https://elibrary.ferc.gov/eLibrary/filelist?accession_number=20240814-5165
[7]At page 10, https://www.sec.gov/ix?doc=/Archives/edgar/data/66756/000006675624000007/ale-20231231.htm
[8] https://elibrary.ferc.gov/eLibrary/filelist?accession_number=20240814-5165
[9] Application, at page 16.
[10] July 19, 2024 Initial Filing – Petition for Approval Docket Number PA-24-198 www.edockets.state.mn.us/edockets/searchDocuments.do?method=showPoup&documentId={20E3CC90-0000-CB1E-9F69-13722A140B90}&documentTitle=20247-208768-01
[11] July 19, 2024 Verified Petition For Declaratory Ruling Or, In The Alternative,
For Approvals Of A Holding Company Takeover Docket No 5820-DR-100 https://apps.psc.wi.gov/ERF/ERFview/viewdoc.aspx?docid=509235
[12] August 26, 2024 Comments of the Citizens Utility Board of Minnesota Docket PA-24-198 www.edockets.state.mn.us/edockets/searchDocuments.do?method=showPoup&documentId={007D6C91-0000-C83D-A721-A9B636DE555E}&documentTitle=20248-209629-02
[13] August 19, 2024 Comments of the Office of the Attorney General Docket PA-24-198 www.edockets.state.mn.us/edockets/searchDocuments.do?method=showPoup&documentId=%7B10186B91-0000-C13B-AB1F-393C704BB6FE%7D&documentTitle=20248-209588-02
[14]Lovrien, Jimmy, Minnesota Attorney General, others concerned over possible Allete sale, Duluth News Tribune, August 30, 2024, www.duluthnewstribune.com/news/local/minnesota-attorney-general-others-concerned-over-possible-allete-sale
You might be interested in
Stay Updated on Public Citizen
Follow Public Citizen
Support Our Work
Crypto’s Protection Racket
By Bartlett Naylor
Download PDF version of comment
In Hollywood crime movies, criminals regularly pay off the cops to protect their illicit trade, plots often rooted in reality. (See Serpico.) The cryptocurrency sector, or “crypto bros,” may be film buffs, because they’re using the very same script. They have deployed a record amount of money into the 2024 election cycle in an attempt to convince lawmakers to ignore crypto’s harms, with obvious results. They’ve wilted the will of politicians, especially Democrats, who must otherwise understand that crypto is a Ponzi-like scheme. (The only way to make a profit is to sell the crypto to someone willing to pay more; there are no dividends from a token that is, after all, simply a string of computer numbers.) The crypto industry’s ultimate target: dismantling the efforts by the Securities and Exchange Commission (SEC), led by brave chair Gary Gensler, to insist that crypto purveyors honor the same rules that apply to all those who invite the public to invest in their projects.
Unfortunately, the current state affairs is that a lawmaker who votes “no” on pro-crypto legislation that would disenfranchise the SEC will lead a handful of crypto zillionaires to deploy millions of dollars to defeat this scrupulous lawmaker in the coming election. Public Citizen’s Rick Claypool documented this phenomenon in two reports. As of August 2024, the crypto sector amassed $169 million to spend in support or opposition to candidates based on their crypto statements and votes. That amount is more than any other sector is spending in elections—more than Wall Street, Big Oil, and Pharma. It’s nearly half of all corporate money contributed in the 2024 election cycle. It’s 15 percent of all known corporate money contributions since the U.S. Supreme Court’s disastrous 2010 decision in Citizens United that made such unlimited corporate political spending legal (expanding the previous 1976 Buckley decision). Alone, this deluge of corporate spending constitutes an unprecedented, frightening corruption of lawmaking, the starkest example to date, as Public Citizen President Robert Weissman has opined.
To be clear, not all corporate political spending is legal. Public Citizen co-filed a complaint with Molly White alleging that contributor Coinbase violated campaign finance rules that prohibit contributions when the firm seeks or obtains a government contract.
A dangerous U.S. House bill that not only harms investor protection for crypto but any investment that uses the same digital platform called blockchain to record transactions drew 71 Democrats in support. These Democrats defied the warnings of Rep. Maxine Waters (D-Calif.) ranking member of the House Financial Services Committee and Minority Leader Hakeem Jeffries (D-N.Y.). Fear of crypto political spending arguably prevailed.
Other illicit enterprises will surely learn from the example. A sector that bilks billions from Americans will find it a cheap escape from accountability to redirect a small fraction of these ill-gotten gains to buy off enough politicians to block sensible reform. Ripple, a crypto sponsor and payment systems firm, paid a $125 million fine following an SEC case. Its otherwise sizeable $45 million in political spending so far in 2024 pales next that penalty.
Importantly, this spending doesn’t pay for political advertisements that discuss crypto. The political ads don’t mention crypto at all. Crypto policy holds little interest for most Americans. Ninety-three percent of Americans neither own nor use crypto at all, according to Federal Reserve data. And that figure rose over the last few years as more people learn about crypto. The number of people who own crypto has declined over the last several years as information about the sector has increased. Maybe these political ads avoid referencing crypto because a growing number of Americans understand this sector as all hype and little hope. A 2022 Pew poll found that about half of all crypto investors report their experience fell below expectations, and only 15 percent said they did better than anticipated. In 2023, Pew found than 88 percent of Americans familiar with crypto reported they were not confident that crypto is safe. In the sober confines of the courtroom, where lies and exaggeration aren’t tolerated, even crypto lawyers liken crypto to a Beanie Baby in order to escape SEC oversight, as opposed to an investment in Beanie Baby Inc. (Technically, Ty Inc. ) Maybe the ads don’t mention crypto because sector leaders such as Sam Bankman-Fried and Changpeng Zhao now serve prison sentences for theft or other lawbreaking connected to their crypto companies. Maybe the political ads funded by crypto avoid mentioning their product because scams abound, as detailed weekly by crypto skeptic Molly White, or recorded on an SEC website.
Instead, crypto political spending goes to advertisements with no mention of the candidate’s crypto position. Crypto political funder Fairshake– underwritten largely by venture capital firm Andreesen Horowitz, crypto firm Coinbase, Ripple and others– spent some $10 million to defeat Senate candidate Katie Porter who challenged the massive energy use by crypto. Instead of this issue, the ads deviously claimed she’d broken a promise to eschew support from disfavored industries such as Big Oil and Big Banks, an unfounded assertion. Hiding their breathless claims of crypto potential tellingly reveals they must understand this neither resonates with voters, nor finds purchase in reality
Perniciously, this crypto spending goes to super PACS legally divorced from candidate campaigns. This insulates the candidate from criticism that they may be shills for the crypto Ponzi scheme. By contrast, candidates that accept money from certain rapacious industries such as Wall Street or Big Pharma face public opprobrium for accepting this lucre, as Fairshake unfairly exploited in the Porter race.
The crypto bros openly discuss this protection racket. Said one, “Having money, unfortunately, is a big part of the way government works in the US.” Said another, “It’s now become quite risky, I would say even maybe political suicide, to be anti-crypto in DC.”
Politicians may believe they face a cruel dilemma. They can avoid taking a position on crypto or even vote to support the sector, betraying their scruples. Or they can oppose crypto, and invite the wrath of crypto-funded ads, jeopardizing their election and ability to serve average Americans on a myriad of other issues. Washington doesn’t always follow the Hollywood script where the honest cop eventually prevails over the corrupt ones.
There’s no easy, obvious escape from this dilemma.
Ultimately, Citizens United must be overturned and campaign finance reform fully realized. Until then, this Hollywood crime show becomes a horror movie series.