Petition to the FDA to Strengthen Safety Warning for Botox and Related Drugs and Remove Misleading Claims From Botox/Botox Cosmetic Labeling
December 12, 2023
Robert M. Califf, M.D. Commissioner, Food and Drug
Administration Department of Health and Human Services
WO 2200
10903 New Hampshire Avenue
Silver Spring, MD 20993-0002
Division of Dockets Management
Food and Drug Administration
Department of Health and Human Services
5630 Fishers Lane, Room 1061
Rockville, MD 20852
On behalf of Public Citizen, a consumer advocacy organization with more than 500,000 members and supporters nationwide, and Public Citizen’s Health Research Group, the undersigned submit this petition under the Federal Food, Drug, and Cosmetic Act (FD&C Act, 21 U.S.C. § 352) and under Food and Drug Administration (FDA) regulations at 21 C.F.R. § 10.30 and § 201.56 to request that the Commissioner of Food and Drugs take action to strengthen the risk warning on the labeling of all approved botulinum toxin biological products (hereafter, BT products). These products are abobotulinumtoxinA (Dysport),[1] daxibotulinumtoxinA-lanm (Daxxify),[2] incobotulinumtoxinA (Xeomin),[3] onabotulinumtoxinA (Botox, Botox Cosmetic),[4],[5] prabotulinumtoxinA-xvfs (Jeuveau),[6] and rimabotulinumtoxinB (Myobloc).[7]
As shown in sections 3.3.2 through 3.3.5 of this petition, there is postmarketing evidence that both cosmetic and therapeutic BT products — even when used at recommended doses, either during initial or subsequent (repeated) treatment — are associated with systemic iatrogenic botulism or related symptoms, which may require prompt administration of botulinum antitoxin to avoid disease progression and possible serious outcomes, including temporary paralysis, hospitalization, and even death. This risk is neither adequately clear nor emphasized in the current labeling of these products. Instead, it is masked with the euphemism of “distant spread of toxin effect” beyond injection sites. Furthermore, the current FDA-approved labeling of BT discusses the need to administer botulinum antitoxin only in the context of excessive dosing, accidental injection, or oral ingestion of these products. Therefore, this petition requests that the FDA make the potential risk of systemic iatrogenic botulism and related symptoms, and the need to administer botulinum antitoxin, clear in the boxed warning on the labeling of BT products.
As discussed in section 4 of this petition, we also request that the agency require the removal of misleading promotional claims from the labeling of onabotulinumtoxinA (Botox, Botox Cosmetic) to avoid misleading clinicians to inject these medications based on the inaccurate mantra that these products are “always safe” and devoid of serious adverse effects.
Overall, implementing these actions will be in the best interest of the public as this will allow clinicians and patients to make more informed decisions about the benefit-risk balance of BT products. It also will help inform patients about how to detect symptoms of toxin spread sooner, facilitating appropriate time-sensitive treatment. This is especially important given the continued surge in the use of these products as therapeutic agents for neuromuscular disorders and for cosmetic medical aesthetics, as well as their growing list of approved indications and their continued uses for unapproved indications.
A. ACTIONS REQUESTED
Promptly require the following
(1) Modify the existing boxed warning in the product labeling of abobotulinumtoxinA (Dysport), daxibotulinumtoxinA-lanm (Daxxify), incobotulinumtoxinA (Xeomin), onabotulinumtoxinA (Botox, Botox Cosmetic), prabotulinumtoxinA-xvfs (Jeuveau), and rimabotulinumtoxinB (Myobloc) to emphasize the risk of severe systemic iatrogenic botulism. We do not distinguish between various approved BT products in this regard because the risk applies to all of them. We suggest the following language to enhance the current boxed warning:
Postmarketing cases of severe systemic iatrogenic botulism and related symptoms — many of which have required hospitalization and treatment with botulinum antitoxin — have occurred within hours to weeks after injection of botulinum-toxin products, including those intended for cosmetic uses. These cases occurred even with recommended doses in patients who had an initial injection as well as in those who had tolerated previous injections of these products. Signs and symptoms of iatrogenic botulism may include asthenia, generalized muscle weakness, diplopia, ptosis, dysphagia, dysphonia, dysarthria, urinary incontinence, and breathing difficulties. Swallowing and breathing difficulties can be life threatening, and there have been reports of death. Therefore, caution should be exercised upon each administration of BT products. Particularly, monitoring and prompt recognition of impending systemic iatrogenic botulism as well as early administration of botulinum antitoxin can be critical to improving patient outcomes associated with these potentially life-threatening adverse events.
Conforming changes also should be made in other sections of the labeling of BT products.
(2) Remove the following misleading promotional paragraph (a) from the labeling of onabotulinumtoxinA (Botox), paragraph (b) from the labeling of onabotulinumtoxinA (Botox Cosmetic), and paragraph (c) from the medication guide of both Botox and Botox Cosmetic:[8],[9]
(a) “No definitive serious adverse event reports of distant spread of toxin effect associated with BOTOX for blepharospasm at the recommended dose (30 Units and below), severe primary axillary hyperhidrosis at the recommended dose (100 Units), strabismus, or for chronic migraine at the labeled doses have been reported.”
(b) “No definitive serious adverse event reports of distant spread of toxin effect associated with dermatologic use of BOTOX /BOTOX Cosmetic at the labeled dose of 20 Units (for glabellar lines), 24 Units (for lateral canthal lines [crow’s feet]), 40 Units (for forehead lines with glabellar lines), 44 Units (for simultaneous treatment of lateral canthal lines and glabellar lines), 64 Units (for simultaneous treatment of lateral canthal lines, glabellar lines, and forehead lines), or 100 Units (for severe primary axillary hyperhidrosis) have been reported.”
(c) “There has not been a confirmed serious case of spread of toxin effect away from the injection site when BOTOX has been used at the recommended dose to treat chronic migraine, severe underarm sweating, blepharospasm, or strabismus, or when BOTOX Cosmetic has been used at the recommended dose to treat frown lines, crow’s feet lines, and/or forehead lines.”
B. STATEMENT OF GROUNDS
1. Legal Standards
1.1. Legal standard applicable to our first requested action
Revising the boxed warning on the labeling of BT products to emphasize the risk of severe systemic iatrogenic botulism:
Inherent in the FDA’s authority to regulate prescription drugs is its authority to require biologics license application (BLA) holders of all BT products to adequately describe drug risks on the labeling of their products. Particularly, the agency can require a boxed warning on the product labeling of prescription drugs for “serious warnings, particularly those that may lead to death or serious injury.”[10] The FDA advises that a boxed warning is used for cases in which there is “an adverse reaction so serious in proportion to the potential benefit from the drug . . . that it is essential that is be considered in assessing the risks and benefits of using the drug [or] [t]here is a serious adverse reaction that can be prevented or reduced in frequency or severity by appropriate use of the drug…”[11]
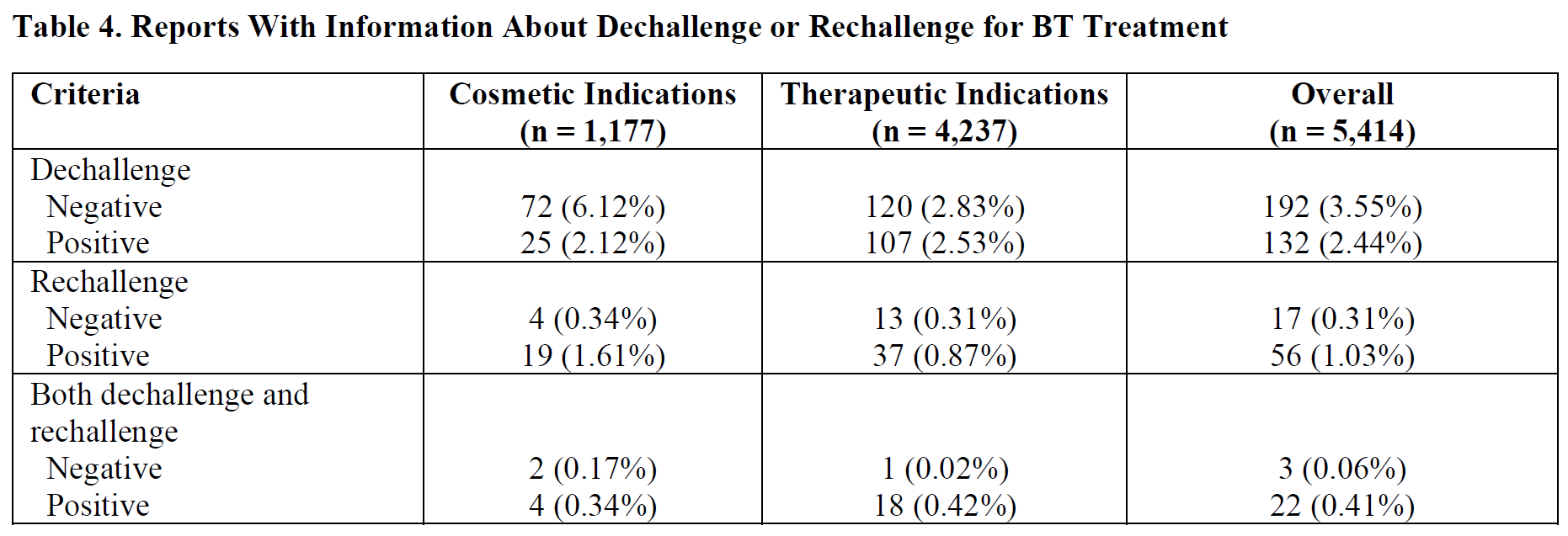
Under federal law, the FDA must require revising drug labeling to include a warning about a clinically significant risk “as soon as there is reasonable evidence of a causal association with a drug; a causal relationship need not have been definitely established.”[12] In assessing evidence of a causal relationship for inclusion in the warnings section of a drug label, the FDA advises considering the following factors: “(1) the frequency of reporting; (2) whether the adverse event rate in the drug treatment group exceeds the rate in the placebo and active-control group in controlled trials; (3) evidence of a dose-response relationship; (4) the extent to which the adverse event is consistent with the pharmacology of the drug; (5) the temporal association between drug administration and the event; (6) existence of dechallenge and rechallenge experience; and (7) whether the adverse event is known to be caused by related drugs.”[13] Importantly, the agency advises that reasonable evidence of a causal association between an adverse event and the use of a particular drug is enough and that a causal relationship need not to be definitively established.
1.2. Legal standard applicable to our second requested action
Removing misleading promotional claims from the labeling of onabotulinumtoxinA (Botox, Botox Cosmetic) about the lack of “definitive” or “confirmed serious” cases of distant spread of toxin at recommended doses for certain specified indications:
According to the FD&C Act, a “drug or device shall be deemed to be misbranded . . . [i]f its labeling is false or misleading in any particular.”[14] Therefore, BLA holders of BT products have an ongoing obligation to ensure that the labeling of their products “must be informative and accurate and neither promotional in tone nor false or misleading in any particular.”[15] When new information becomes available that causes information on product labeling to be “inaccurate, false, or misleading,” these BLA holders must take steps to change the content of their labeling. Furthermore, it is imperative for the agency to mandate the removal of misleading promotional claims from the labeling of BT products, where applicable, to avoid misleading clinicians and patients.
2. Background
2.1. Approved BT products and their indications
Produced by the anaerobic, gram-positive bacterium Clostridium botulinum, there are seven BT types: A to G.[16] These BT types are highly toxic protein neurotoxins that are distinct but structurally similar to each other. BT types A and B have been purified and diluted for commercial use as injectable biologics. When injected intramuscularly for therapeutic uses, BT products target receptor binding sites on motor nerve terminals and inhibit the release of acetylcholine (the principal neurotransmitter at the neuromuscular junction).[17] This action causes partial chemical denervation of affected muscles and results in a reduction of muscle activity. The onset of muscle weakness due to BT products usually occurs within days of injection and lasts from three to six months on average.[18] The effect of BT products is temporary because axonal sprouting and new neuromuscular junctions may form later. Therefore, treatment frequency usually is determined based on individual patient response. However, dosing intervals that are fewer than three months are not recommended because of the risk of developing neutralizing antibodies, leading to decreased effectiveness of, or resistance to, BT products.
Doses of FDA-approved BT products are measured in units of biologic activity. One unit of a BT product corresponds to the lethal dose that kills half of a group of experimental mice (LD50).[19] The potencies of BT products from different BLA holders differ significantly due to the use of distinct manufacturing methods.[20] Therefore, these products also are neither bioequivalent nor interchangeable. Nonetheless, some dose conversion factors for each BT product have been reported.
In 1989 and 2002, the FDA approved two formulations of onabotulinumtoxinA (Botox, Botox Cosmetic) for therapeutic and cosmetic indications, respectively.[21],[22] In 2009 and 2010, the Agency approved abobotulinumtoxinA (Dysport),[23] and incobotulinumtoxinA (Xeomin), respectively,[24] both of which also are approved now for therapeutic and cosmetic indications. Since 2000, the agency has approved rimabotulinumtoxinB (Myobloc) for therapeutic indications only.[25] In 2019, the FDA approved prabotulinumtoxinA-xvfs (Jeuveau) for cosmetic indications only in 2019.[26] Most recently, the agency approved daxibotulinumtoxinA-lanm (Daxxify) for cometic indications in 2022 and for cervical dystonia in 2023.[27] All of these approved BT products are type A botulinum toxins, except for rimabotulinumtoxinB, which is a type B botulinum toxin.
Initially, the FDA approved onabotulinumtoxinA to treat two rare eye-muscle disorders (blepharospasm and strabismus) in adults. Since then, the agency has expanded the collective list of approved therapeutic indications of BT products to treat the following additional conditions and age groups (note, not all BT products are approved for all these indications):
- cervical dystonia in adults to reduce the severity of abnormal head position and neck pain;
- chronic sialorrhea in adults;
- lower- and upper-limb spasticity (including that associated with cerebral palsy) in patients two years of age and older;
- neurogenic detrusor overactivity in patients five years of age and older who have an inadequate response to (or are intolerant of) anticholinergic medication;
- overactive bladder with symptoms of urge urinary incontinence, urgency, and frequency, in adults who have an inadequate response to (or are intolerant of) an anticholinergic medication;
- prophylaxis of chronic migraine in adults;
- severe primary axillary hyperhidrosis that is inadequately managed by topical agents in adults;
- urinary incontinence due to detrusor overactivity associated with neurologic conditions in adults who have an inadequate response to (or are intolerant of) an anticholinergic medication.
The FDA initially approved onabotulinumtoxinA for a single cosmetic indication: temporary improvement in the appearance of moderate-to-severe glabellar (frown) lines associated with corrugator or procerus muscle activity in older adults. The agency eventually removed the age restriction of this indication. Subsequently, the FDA also has approved incobotulinumtoxinA, prabotulinumtoxinA-xvfs, and daxibotulinumtoxinA-lanm for temporary improvement of moderate-to-severe glabellar lines in adults. In recent years, the FDA has expanded the approval of onabotulinumtoxinA to include two additional indications:
- temporary smoothing of moderate-to-severe lateral canthal lines associated with orbicularis oculi activity in adults;
- temporary smoothing of moderate-to-severe forehead lines associated with frontalis muscle activity in adults.
The Appendix shows the recommended single-session dosages per approved indication for each approved BT product.
Importantly, off-label uses of BT products are common and span a wide-range of dermatologic, gastrointestinal, neurologic, and other conditions as well as various cosmetic uses, often without evidence from controlled clinical trials,[28] leading to substantial patient harm. For example, Allergan paid a $600 million fine in 2010 due to its marketing of onabotulinumtoxinA for unapproved uses and pleaded guilty to a misdemeanor charge of “misbranding.”[29] This fine was a settlement with federal prosecutors who accused the company of “illegally, vigorously and without any thought to the possible negative health effects to which it subjected patients, promoted off-label uses” of onabotulinumtoxinA.[30]
The global market of BT products stood at $5.9 billion in 2022 and is projected to reach nearly $13 billion in 2033, with a compound annual growth rate of 7.1%.[31] As an example of the surging popularity of these products, BT type A injections were the top non-surgical cosmetic procedure in the U.S. in 2020, with 4.4 million injections (94% of which were in women) performed that year.[32] Notably, 12,767 of those injections occurred in individuals age 13 to 19, 64,088 were injected in individuals age 20 to 29, and 811,314 were injected in individuals age 30 to 39. These numbers, indicating growing “preventive” cosmetic uses of BT products[33] come as no surprise as their BLA product holders, like Allergan, have expanded their marketing strategy to target millennials[34] and men.[35]
2.2. Pertinent regulatory history of BT products
In our January 2008 citizen petition to the FDA regarding BT products, we noted that the labeling of these products included inconspicuous, “scattered” information regarding the risk of distant spread of the toxin beyond injection sites, resulting in serious adverse events including aspiration pneumonia (that sometimes leads to death), dysphagia, and muscle weakness.[36] Therefore, we asked the agency to require a boxed warning on the labeling of BT products to consistently emphasize that distant spread of the toxin is possible and can lead to fatal outcomes. Our petition included results from our analysis of FDA Adverse Event Reporting System (FAERS) data from November 1997 through December 2006, which identified 180 related case reports that were associated with aspiration, dysphagia, or pneumonia, including 87 hospitalizations and 16 deaths (4 of those deaths occurred in children under the age of 18).
In February 2008, the FDA acknowledged receipt of botulism reports in association with BT products, noting that potentially life-threatening systemic toxicity can result from local injection in patients with underlying conditions (such as those with cerebral palsy-associated limb spasticity).[37] The agency also announced that it had started to investigate the safety of BT products. However, it was not until April 2009 that the FDA granted our petition in part and indicated that it had identified a total of 225 case reports with signs and symptoms that are suggestive of “potential iatrogenic botulism.”[38] These reports were consistent with “the pharmacologic effects of botulinum toxin at sites noncontiguous and distant from the site of injection” after the use of BT products for both approved and unapproved uses. The agency identified those cases from data submitted by BLA holders of BT products (Allergan and Solstice), its review of the literature, and its own FAERS analysis. As part of its FAERS search, the agency used botulism and certain other reactions as key MedDRA preferred terms. The agency noted that the mechanism by which distant spread of the toxin occurs has not been established, yet acknowledged that any spread of the toxin beyond the intended site of action can cause serious adverse effects.
The agency also conducted a separate FAERS search associated with a “death” outcome, identifying 58 cases, 17 of which were suggestive of potential iatrogenic botulism. It noted that, although most of these 17 reports involved patients who had underlying neuromuscular disorders with preexisting choking, dysphagia, or pneumopathies (pulmonary disorders), a potential association between the use of BT products and iatrogenic botulism-related symptoms could not be ruled out. These cases reported a temporal relationship between administration of BT and one or more symptoms (including muscle paralysis, dysphagia, and subsequent fatal respiratory failure) that were consistent with chemical denervation associated with BT products, according to the agency. Given that the patients suffered from complications related to other underlying conditions, the agency could not determine whether the administration of BT was a causal factor in the outcome of death. Yet, as an important part of granting our petition in 2009, the FDA mandated adding a boxed warning on all labeling of BT products marketed in the U.S.[39] The current wording of this warning is as follows:[40]
WARNING: DISTANT SPREAD OF TOXIN EFFECT
Postmarketing reports indicate that the effects of [a specific BT product, such as onabotulinumtoxinA] and all botulinum toxin products may spread from the area of injection to produce symptoms consistent with botulinum toxin effects. These may include asthenia, generalized muscle weakness, diplopia, ptosis, dysphagia, dysphonia, dysarthria, urinary incontinence and breathing difficulties. These symptoms have been reported hours to weeks after injection. Swallowing and breathing difficulties can be life threatening and there have been reports of death. The risk of symptoms is probably greatest in children treated for spasticity but symptoms can also occur in adults treated for spasticity and other conditions, particularly in those patients who have underlying conditions that would predispose them to these symptoms. In unapproved uses and in approved indications, cases of spread of effect have been reported at doses comparable to those used to treat cervical dystonia and spasticity and at lower doses…
The FDA also required BLA holders of BT products to send warning letters to doctors who prescribe or inject these products and to produce a medication guide to be given to patients at the time of injection about the distant spread risks of these products. Since then, U.S. medication guides for BT products have been carrying the following warning regarding the risk of “botulism”:
Spread of toxin effects. In some cases, the effect of botulinum toxin may affect areas of the body away from the injection site and cause symptoms of a serious condition called botulism. The symptoms of botulism include:
- loss of strength and muscle weakness all over the body
- double vision, blurred vision and drooping eyelids
- hoarseness or change or loss of voice (dysphonia)
- trouble saying words clearly (dysarthria)
- loss of bladder control
- trouble breathing
- trouble swallowing
These symptoms can happen hours to weeks after you receive an injection of [BT products]. These problems could make it unsafe for you to drive a car or do other dangerous activities.
In addition, a variation of the following current overdose section has been part of U.S. BT-product labeling:
OVERDOSAGE
Excessive doses of [a specific BT product, such onabotulinumtoxinA] may be expected to produce neuromuscular weakness with a variety of symptoms.
Symptoms of overdose are likely not to be present immediately following injection. Should accidental injection or oral ingestion occur or overdose be suspected, the person should be medically supervised for several weeks for signs and symptoms of systemic muscular weakness which could be local, or distant from the site of injection… These patients should be considered for further medical evaluation and appropriate medical therapy immediately instituted, which may include hospitalization.
If the musculature of the oropharynx and esophagus are affected, aspiration may occur which may lead to development of aspiration pneumonia. If the respiratory muscles become paralyzed or sufficiently weakened, intubation and assisted respiration may be necessary until recovery takes place. Supportive care could involve the need for a tracheostomy and/or prolonged mechanical ventilation, in addition to other general supportive care.
In the event of overdose, antitoxin raised against botulinum toxin is available from the Centers for Disease Control and Prevention (CDC) in Atlanta, GA. However, the antitoxin will not reverse any botulinum toxin-induced effects already apparent by the time of antitoxin administration. In the event of suspected or actual cases of botulinum toxin poisoning, please contact your local or state Health Department to process a request for antitoxin through the CDC. If you do not receive a response within 30 minutes, please contact the CDC directly at 1-770-488-7100. More information can be obtained at http://www.cdc.gov/mmwr/preview/mmwrhtml/mm5232a8.htm.
3. Requested Action 1: Risk of severe systemic iatrogenic botulism
3.1. Action 1 and its rationales
Promptly require the following:
(1) Modify the existing boxed warning in the product labeling of abobotulinumtoxinA (Dysport), daxibotulinumtoxinA-lanm (Daxxify), incobotulinumtoxinA (Xeomin), onabotulinumtoxinA (Botox, Botox Cosmetic), prabotulinumtoxinA-xvfs (Jeuveau), and rimabotulinumtoxinB (Myobloc) to emphasize the risk of severe systemic iatrogenic botulism. We do not distinguish between various approved BT products in this regard because the risk applies to all of them. We suggest the following language to enhance the current boxed warning:
Postmarketing cases of severe systemic iatrogenic botulism and related symptoms — many of which have required hospitalization and treatment with botulinum antitoxin — have occurred within hours to weeks after injection of botulinum-toxin products, including those intended for cosmetic uses. These cases occurred even with recommended doses in patients who had an initial injection as well as in those who had tolerated previous injections of these products. Signs and symptoms of iatrogenic botulism may include asthenia, generalized muscle weakness, diplopia, ptosis, dysphagia, dysphonia, dysarthria, urinary incontinence, and breathing difficulties. Swallowing and breathing difficulties can be life threatening, and there have been reports of death. Therefore, caution should be exercised upon each administration of BT products. Particularly, monitoring and prompt recognition of impending systemic iatrogenic botulism as well as early administration of botulinum antitoxin can be critical to improving patient outcomes associated with these potentially life-threatening adverse events.
Conforming changes also should be made in other sections of the labeling of BT products.
As described in section 2.2. of this petition, in 2009, 20 years after its initial approval of the first BT product (onabotulinumtoxinA) for therapeutic uses, the FDA announced that it had finished its literature review and FAERS analysis regarding the safety of BT products and mandated the addition of a boxed warning on the labeling of BT products. The content of this boxed warning remains largely unchanged since then.
There are three shortcomings of this boxed warning. First, although the FDA noted in 2009 that it had identified 225 case reports that it characterized as suggestive of “potential iatrogenic botulism” associated with the use of BT products, it did not require the explicit mention of the risk of “severe systemic iatrogenic botulism” in the boxed warning. Instead, the agency opted to require including a euphemism for botulism: “distant spread of toxin effect.” Specifically, the warning notes that these medications “may spread from the area of injection to produce symptoms [such as asthenia, generalized muscle weakness, diplopia, ptosis, dysphagia, dysphonia, dysarthria, urinary incontinence, and breathing difficulties that are] consistent with botulinum toxin” effects. However, all these symptoms are those of iatrogenic botulism. In fact, the “botulism” term is not mentioned anywhere in the prescribing information of the labeling of BT products and is mentioned only once in the medication guide for patients, where it is buried toward the end. It is not publicly known whether this omission was negotiated between the FDA and BT product makers. In fact, Allergan had previously requested removal of the word “botulism” from the boxed warning, according to publicly available legal notes dating to 2013 and stemming from an Oklahoma District Court lawsuit involving a child who developed botulism after BT treatment.[41] Notably, this case was settled during trial “while the child’s treating physician was testifying,” according to the attorney representing this child.[42]
Second, the risk of distant spread of BT products in the boxed warning is emphasized only in the context of spasticity treatment (especially in children) and patients with certain other underlying conditions. Although the current warning acknowledges that distant spread has occurred in “approved indications… at doses comparable to those used to treat cervical dystonia and spasticity and at lower doses,” it fails to explicitly state that there is evidence that iatrogenic botulism and botulism-related symptoms have occurred after injections of recommended BT doses at both initial and subsequent BT injections for various indications. Notably, in its assessment of prabotulinumtoxinA-xvfs (sold under the brand name Nuceiva in Europe), the European Medicines Agency indicates that, although distant spread of BT toxin effects are uncommon with the use of this BT product, they increase after repeated treatment with it.[43] Furthermore, the special warnings and precautions for use section in the UK labeling of onabotulinumtoxinA — which addresses the potential for overdose and serious adverse effects related to BT spread — advises that adverse effects “can occur despite previous injections being well tolerated. Caution should therefore be exercised on the occasion of each administration.”[44]
Third, although the boxed warning mentions that the distant spread of BT products can cause “[s]wallowing and breathing difficulties [which] can be life threatening” and that “there have been reports of death” associated with this risk, it does not include any information about botulinum antitoxin, whose early administration is critical for the management of severe systemic iatrogenic botulism caused by the distant spread of the toxin. Even the general warnings section regarding BT overdose limits the need for administering botulinum antitoxin to the context of excessive dosing, accidental injection, or oral ingestion of these products.
In the following sections, we provide an overview of iatrogenic botulism and present evidence supporting our request for FDA action regarding the risk of systemic iatrogenic botulism. This evidence is meant to build on, and update, the FDA’s 2009 evidence review.
3.2. Background information on iatrogenic botulism
3.2.1. Overview of botulism and iatrogenic botulism
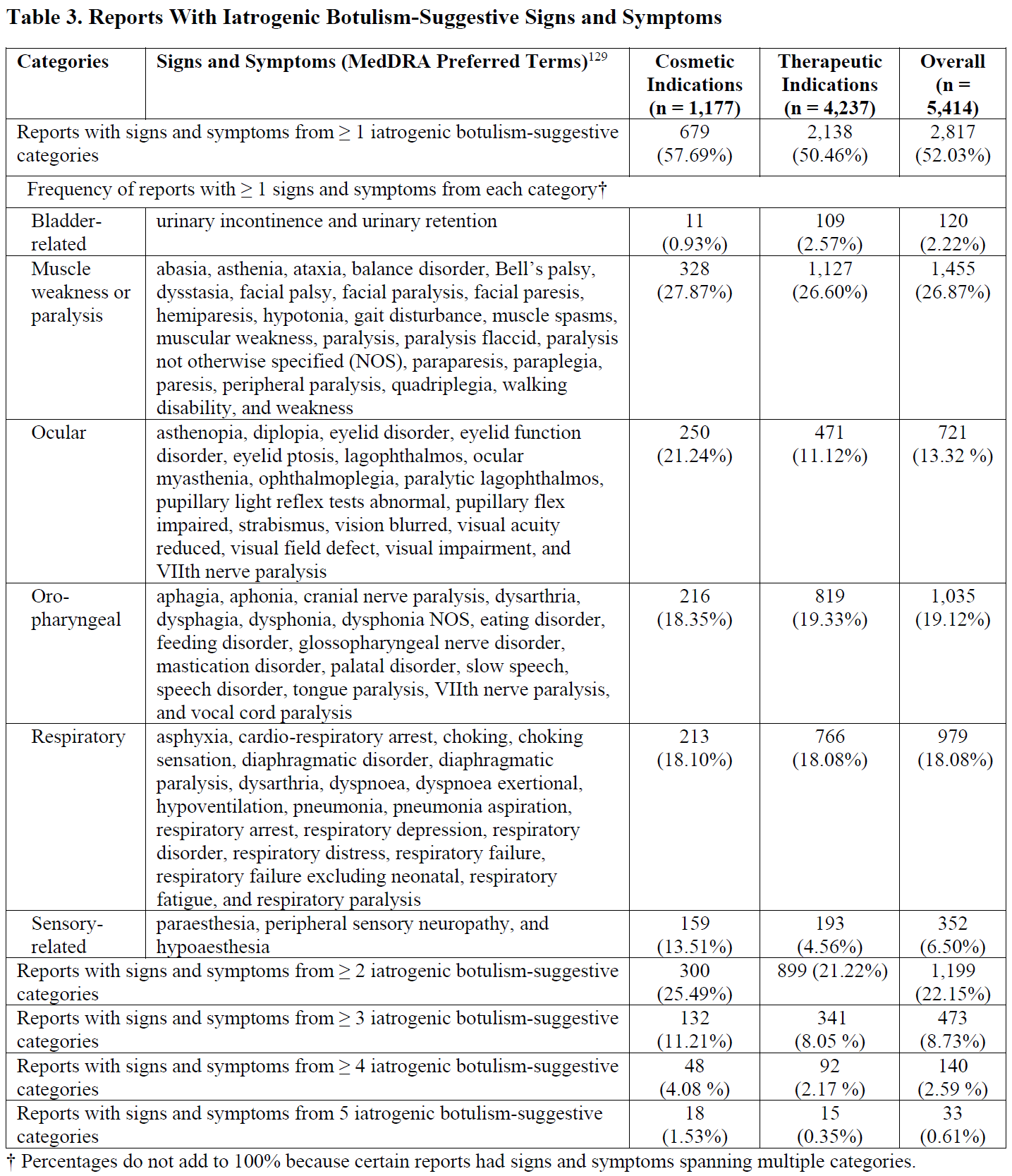
Botulism is a rare but serious and potentially fatal neuroparalytic illness caused by the action of botulinum toxins. Most physicians have never seen a case of botulism.[45] This illness is considered a medical emergency because of its severity and because time is of the essence for its management. Noninfant botulism is categorized by mode of transmission and toxin production into four main forms: classic or foodborne, intestinal (the adult variant of infant botulism), wound, and iatrogenic botulism. Reports of iatrogenic botulism — a complication of treatment with BT products due to their potential for diffusion and systemic spread beyond injection sites — have increased in recent years, mirroring the rising popularity of these products.[46]
Each botulism form is characterized by different exposure routes, but all forms share certain common clinical features. According to CDC guidelines, classic symptoms of botulism usually involve minor visual changes, followed by progressive cranial palsies, which may be followed by bilateral descending flaccid paralysis.[47] In various patients, the range of neurologic signs might span from only ptosis or mild cranial nerve abnormalities to bilateral descending flaccid paralysis, involving axial, extremity, and respiratory muscles. Per the CDC, the most reported signs and symptoms of noninfant botulism in the U.S. include blurred vision, diplopia or ptosis, dilated pupils, dry mouth, dysphagia, shortness of breath, difficulty speaking (slurred speech, trouble speaking clearly, or dysarthria), changes in speech (including hoarseness, nasal speech, or dysphonia), and weakness or fatigue as well as descending paralysis.
Importantly, botulism patients tend to be afebrile and usually are alert. These patients also do not experience cognitive deficits. However, features of the disease (including facial paralysis, ophthalmoplegia, slurred speech, and inability to move due to muscle weakness) might lead people to think that these patients are experiencing an altered mental status or are comatose, despite the fact they are aware and comprehend what is happening around them.
For iatrogenic botulism, the body tissues adjacent to the injection site may be the only, most, or earliest affected from the diffusion of the toxin, depending on the severity of the condition.[48] Subsequently, general muscle weakness, velo-pharyngeal insufficiency and dysarthria, dysphonia, dysphagia, ptosis, diplopia, respiratory distress, and urinary incontinence may ensue.[49] In fact, the signs and symptoms of iatrogenic botulism, as presented under the risks of distant toxin spread in the current boxed warning of BT products, greatly overlap with most reported signs and symptoms of noninfant botulism in the U.S.
As with other forms of noninfant botulism, patients with iatrogenic botulism may present with atypical symptoms, such as asymmetric muscle weakness or asymmetric ptosis, which are signs and symptoms of asymmetric cranial nerve involvements.[50],[51] Cosmetic botulism can be subtle and often is not detected early,[52] leading to delayed or incorrect alternative diagnosis of this condition. Importantly, the incubation period for iatrogenic botulism can be delayed, with initial signs and symptoms mostly presenting within hours to weeks after BT product injection.
Due to their high potency, there are no pharmacokinetic studies of BT products in humans.[53] Nonetheless, diffusion of BT products from the injected muscles to distant muscles in humans has been documented for decades.[54] Evidence from animal experiments shows that BT type A can move from peripheral sites to more centrally located motor neurons via retrograde transport.[55] Recent in-vitro evidence suggests that the toxin can spread within networks of neurons resulting in long-distance effects.[56] However, the mechanism responsible for the diffusion of BT products from injected sites is not fully understood yet.
3.2.2. Diagnosis of systemic iatrogenic botulism
There are no specific guidelines for the diagnosis of iatrogenic botulism; rather, it is largely diagnosed based on a history of recent BT-product injection and — just as with other forms of botulism — a high degree of suspicion of this condition, based on a thorough neurological examination.[57] Prompt recognition of systemic iatrogenic botulism is critical because botulinum antitoxin — the only specific therapy for botulism — must be administered to patients as quickly as possible.
The only FDA-approved laboratory test for identifying botulism, including iatrogenic botulism, is the mouse bioassay (MBA). The MBA test is only available in reference laboratories because it necessitates maintenance of mouse colonies and expertise in recognizing botulism signs in mice. It is used to confirm botulism and its results can be available within 24 hours. However, the CDC cautions that the test is not highly sensitive because “low levels of toxin that are sufficient to produce human illness might not produce signs in mice.” A highly sensitive botulism test called the matrix-assisted laser desorption/ionization time-of-flight mass spectrometry (MALDI-TOF MS) is not commonly available and is time-consuming, but has been used to support the diagnosis of iatrogenic botulism in MBA-negative specimens.[58]
Electrophysiological studies are helpful for supporting the diagnosis of iatrogenic botulism, but there are few reports of them.[59] Findings that are suggestive of botulism from these studies include marked abnormality in distant and proximal muscles (detected by needle electromyography [EMG]),[60] increased jitter and blocking phenomena, indicative of a dysfunction of the neuromuscular junction (detected by single-fiber EMG),[61],[62] and signs of recent denervation without signs of reinnervation (detected by a neuromuscular biopsy).[63] Notably, there is evidence that local injection of BT affects neuromuscular transmission in distant muscles. For example, single-fiber EMG performed at a distant site from BT injections in five patients showed jitter and blocking, suggesting a presynaptic process, even in the absence of clinical weakness or abnormal routine electrophysiologic testing.[64]
Importantly, some cases of iatrogenic botulism may not be recognized either because of misdiagnosis or because mildly affected individuals may not seek medical care,[65] especially those whose symptoms occur at anatomically remote sites from the injection and those with a time lag between BT injections and the manifestation of botulism symptoms.[66] This occurs because patients may not attribute their anatomically remote or temporally delayed symptoms to BT treatment. Although these symptoms are listed on the labeling of BT products, many clinicians also may fail to attribute them to BT treatment. Common misdiagnoses of iatrogenic botulism include Guillain-Barré syndrome and myasthenia gravis, both of which manifest in flaccid paralysis like iatrogenic botulism.[67]
3.2.3. Management of iatrogenic botulism
Generally, as with other forms of botulism, patients with suspected iatrogenic botulism should always be hospitalized and monitored closely.[68] U.S. clinicians tending to these patients should immediately consult emergency staff at their state public health departments to provide clinical consultations, facilitate antitoxin delivery to treat suspected iatrogenic botulism patients (especially those with a rapidly progressing clinical picture), and help with laboratory testing and interpretation, as necessary.[69] Postponing such consultation can delay the administration of botulinum antitoxin, which is most effective early in the course of the disease to prevent disease progression, including respiratory failure and temporary paralysis. Because laboratory confirmation of botulism may take several days, antitoxin administration should be instituted based on clinical suspicion and consultation with public health officials.
Produced from the serum of toxoid- and toxin-immunized horses and stocked by the CDC, heptavalent botulinum antitoxin (HBAT) is currently the only botulinum antitoxin product for treating noninfant botulism in the U.S.[70] CDC guidelines do not recommend giving patients with suspected botulism a second dose of HBAT unless the suspicion for botulism is high and the neurological signs of this condition worsen after the initial dose should have already taken effect.[71] These guidelines also recommend considering alternative diagnoses if the neurological signs progress for more than a day after the administration of the initial HBAT dose.
HBAT contains antibodies that neutralize all free forms of the seven BT types.[72] However, it does not affect toxins already bound to the presynaptic neurons[73] and does not reverse paralysis, respiratory compromise, or other BT-induced effects already apparent by the time of its administration.[74] However, HBAT can make the illness from botulism less severe, by preventing the progression of symptoms, including by decreasing the duration and extent of paralysis. Therefore, CDC guidelines recommend that HBAT be administered as early as possible in the course of illness in severe or rapidly progressive cases (within 48 hours, and preferably, within 24 hours of symptom onset).[75] For example, researchers from the CDC and the Los Angeles County Department of Health described a patient who developed systemic symptoms of iatrogenic botulism within five days of receiving a recommended dose of BT type A treatment and indicated that “it is unknown whether [this] patient’s dysphagia and dysphonia, which are localized effects expected from [BT] administration, would have progressed to systemic signs and symptoms [of iatrogenic botulism] had HBAT not been administered.”[76]
However, the window of effectiveness for botulinum antitoxin in iatrogenic botulism is unknown.[77] Some published reports showed that clinical improvement occurred when botulinum antitoxin was belatedly administered after the onset of iatrogenic botulism symptoms. For example, a five-year-old child with cerebral palsy who developed iatrogenic botulism symptoms (mainly muscle weakness and ptosis) after receiving 500 units of abobotulinumtoxinA for lower-limb spasticity improved dramatically after receiving three doses of botulinum antitoxin on multiple days starting on the 10th day after onset of symptoms.[78] Similarly, a 29-year-old female showed prompt clinical improvement following belated treatment with HBAT (beginning on the 12th day) after she developed initial symptoms of systemic iatrogenic botulism.[79] This patient had developed these symptoms after she was treated with 600 units of abobotulinumtoxinA to treat palmar and plantar hyperhidrosis in addition to cosmetic treatment. According to the authors, the post-botulinum antitoxin improvement in this patient’s iatrogenic botulism-related symptoms (bilateral ptosis, prominent diplopia in vertical gaze, hypophonia, dysphagia, widespread muscle weakness in her proximal lower- and upper-extremity muscles, and in her head flexion) resulted in her discharge from the hospital 18 days later.
Furthermore, a case report describing two adult women who received off-label injections of BT type A products (most likely onabotulinumtoxinA) in beauty shops in Asia also supports the late administration of botulinum antitoxin in cases of iatrogenic botulism, if patients seek treatment late.[80] Specifically, although the patients in this report had developed several iatrogenic botulism symptoms (mainly diplopia, dysarthria, dysphagia, and bilateral weakness in all limbs) after their BT injections, they did not receive several doses of monovalent type A botulinum antitoxin (a formulation that was formerly distributed by the CDC)[81] until several days after the onset of their iatrogenic botulism symptoms: on the seventh day in the case of one patient and on the ninth day in the second patient. Both patients experienced improvement in their symptoms afterwards. The authors of this case report speculated that what may explain the clinical improvement, and possibly the cessation of symptom progression, after the late antitoxin treatment in these iatrogenic botulism patients is that their neuromuscular junction receptors were not fully saturated with BT.
For patients with localized signs and symptoms of iatrogenic botulism, it has been suggested that clinicians — in close consultation with public health officials — might consider monitoring these patients and only administering the antitoxin should further signs and symptoms of neurologic weakness occur.[82] In other words, patients who do not experience worsening of their symptoms may not need treatment with antitoxin.[83]
Importantly, the CDC guidelines indicate that almost all botulism patients can survive — even without the botulinum antitoxin — if they receive supportive care, as needed.[84] Therefore, close monitoring of the respiratory function of suspected iatrogenic botulism patients is necessary to identify early signs of respiratory failure, which is the major cause of death in affected individuals at the acute stage of the disease. When needed, intubation should be performed in case of respiratory compromise.
Finally, iatrogenic botulism patients who develop flaccid paralysis — which can last from weeks to months — generally require prolonged periods of hospitalization to receive various forms of supportive care, such as nutritional support, until their botulism-induced deficits have resolved. Later during the course of iatrogenic botulism, death is usually caused by complications from protracted intensive care, such as ventilator-associated pneumonia and deep vein thrombosis. Therefore, survival and recovery in patients with severe systemic iatrogenic botulism may necessitate extended use of intensive care resources, a major concern if many patients develop botulism.
3.3. Evidence supporting requested action regarding iatrogenic botulism warning
As discussed in section 3.1. of this petition, the current boxed warning of BT products fails to mention the potential risk of systemic iatrogenic botulism and related symptoms explicitly; instead, it waters down and obscures this serious risk by limiting its wording to the “distant spread of toxin” euphemism. This warning also fails to explicitly acknowledge that the potential risk of systemic iatrogenic botulism can occur in patients receiving initial recommended doses as well as those receiving subsequent recommended doses (i.e., those who have tolerated previous doses) of BT products for various indications. Although this risk appears to be associated with therapeutic uses of BT products, it also can occur with cosmetic uses of these products. Additionally, the current overdose warning of BT products limits the suggested use of botulinum antitoxin administration to cases involving excessive dosing, accidental injection, or oral ingestion of these products.
These omissions need to be promptly corrected because, even though the risk of systemic iatrogenic botulism with the use of these products is potentially small, it is hard to predict which patients are at risk. It is quite possible that any person receiving any dose of these products can be at risk of systemic iatrogenic botulism, although this risk may be smaller at low doses.
Therefore, clinicians and patients alike need to be aware of this potential risk so they can make informed benefit-risk assessments, and therefore decisions, regarding these increasingly commonly used products.
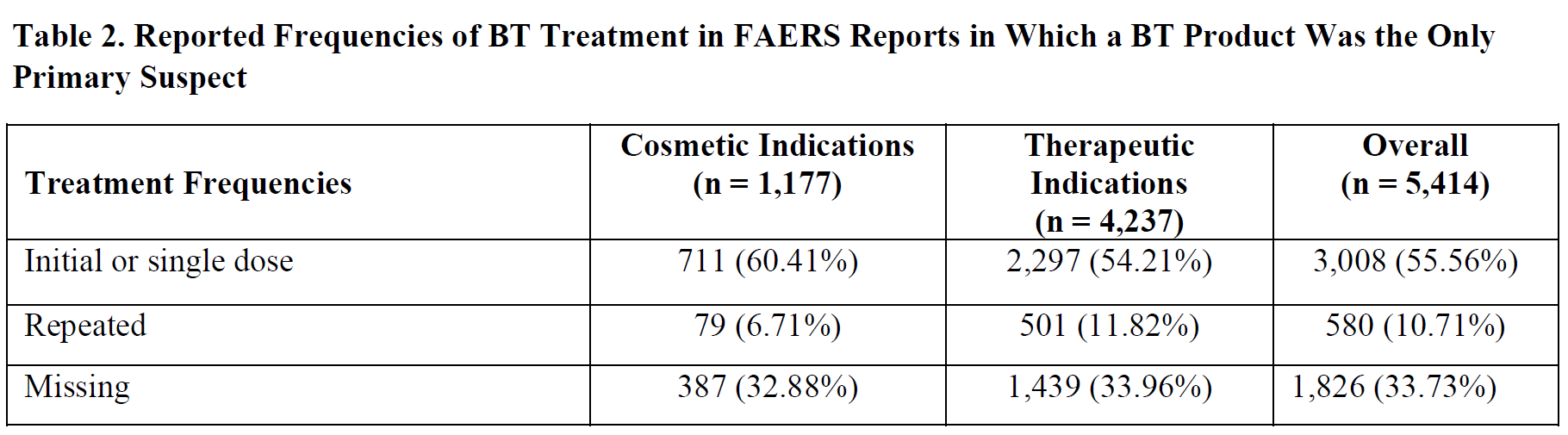
In the next part of this section, we point out the limitations of the safety data available from randomized controlled trials (RCTs) that supported the approval of BT products because these limitations may explain why the risk of systemic iatrogenic botulism was not evident in these trials. Then, we present information from published case reports, case series, and pharmacovigilance studies demonstrating the risk of systemic iatrogenic botulism with recommended initial or subsequent (i.e., repeated) doses of BT products. Finally, we present results from our analysis of the FAERS database lending support for the requested warning related to iatrogenic botulism.
3.3.1. Limited safety evidence from clinical trials
Although RCTs supporting the approval of BT products are supposed to provide critical evidence regarding these products, they are not a good source of uncommon adverse events. This is because these trials were primarily designed and powered to assess the efficacy of BT products.[85] Therefore, the reporting of safety outcomes tended to be less detailed in these trials, which limits the amount of data that could be gleaned from them. In addition, several of these trials involved passive monitoring of safety outcomes (i.e., adverse events were only recorded if patients voluntarily reported them) instead of active monitoring (i.e., specifically prompting or instructing patients to report safety outcomes).
In general, systematic reviews by the Cochrane Collaboration point out that the available RCTs of BT products were predominantly industry-funded — representing a “high risk of bias” — and that they included “an enriched population with a higher probability of benefiting from this therapy,”[86],[87] in the sense that these trials excluded patients with poor responses to BT treatment. Authors of these reviews characterized the short-term safety evidence from these trials as “low” or “moderate” and cautioned about a lack of evidence for drawing definitive conclusions regarding the optimal treatment intervals and doses of BT products, because most available evidence from RCTs evaluated the effect of a single BT product treatment session. Meanwhile, most serious adverse events from BT products are derived from case reports and studies that used data from spontaneous reporting systems.[88]
3.3.2. Evidence from case reports and case series regarding systemic iatrogenic botulism with high BT doses
Prior to the FDA’s 2009 boxed-warning requirement regarding the potential distant spread of botulinum toxin, several case reports had demonstrated systemic spread effects of the toxin consistent with iatrogenic botulism after treatment with these products, including in patients who had previously tolerated earlier treatment with them. Some of these cases involved injections of high doses of these products for therapeutic indications — such as cerebral palsy, which was not an approved indication of these products in the U.S. at the time — and hyperhidrosis.[89],[90],[91],[92],[93],[94] This led some researchers to advise clinicians to be mindful of the “small but potential risk for iatrogenic systemic botulism in patients who previously tolerated local injections” of the same BT product.[95] Some researchers also suggested starting with the smallest dose in BT-naive patients,96[96] a recommendation that is currently included in the labeling of these products.
In 2010, Crowner et al. published a case series article that was funded by the National Institutes of Health.[97] This article described three patients who developed systemic spread of onabotulinumtoxinA in the setting of therapeutic injections. The ages of these patients ranged from 16 to 38 years and their onabotulinumtoxinA doses ranged from 650 to 760 units (from 8 to 13 units/kilogram [kg]). The distant effects on muscles were confirmed with EMG findings, indicating severe, systemic adverse outcomes. These patients had all tolerated previous injections of the same doses, which make predicting such adverse effects “nearly impossible,” according to the authors. Based on additional evidence from their review of the literature, the authors concluded that the risk of developing systemic effects from BT treatment does not seem to be related to its dose based on body weight. Rather, they speculated that this risk is related to total injection dose and injection frequency. The authors suggested that their three cases show that injections of greater than 600 units of onabotulinumtoxinA with follow-up injections every three months may lead to an increased risk of systemic effects. Therefore, they recommended careful consideration of reinjection frequency if greater than 600 units of onabotulinumtoxinA are injected in single sessions. They also called for further studies to examine total injection dose and reinjection frequency of BT treatment.
Consistent with Crowner et al. 2010, there have been other case reports of signs and symptoms of systemic iatrogenic botulism associated with the use of higher-than-recommended doses of BT products for approved therapeutic indications despite previous uneventful therapy with these products.[98],[99] For example, Haddad et al., 2012 described a case of a 24-year-old woman with spinal cord injury who had detrusor overactivity and was treated effectively for a year by antimuscarinics and intradetrusor (bladder-wall muscle) BT injections.[100] Ten days after her last injection with 300 units of onabotulinumtoxinA, she became unable to manipulate the steering wheel of her car and to transfer (e.g. from chair to bed), and developed generalized muscle weakness. Her isometric muscle testing showed an increase in muscle deficits in her triceps and a loss of up to 72% of the strength in her deltoid muscles. Her electrophysiological tests showed jitter in the neuromuscular junctions in her orbicularis oculi muscles. She was diagnosed with botulism-like syndrome after other diagnoses were ruled out. Her clinical and electrophysiological findings normalized within a year.
Para et al., 2020 described a case of a 19-year-old man who developed rapid ascending paralysis and respiratory distress just a day after receiving his first injection of 600 units of onabotulinumtoxinA to treat lower-limb spasticity associated with cerebral palsy.[101] This patient was intubated for airway protection, received treatment with HBAT, and was admitted to the ICU. After five days, he was transitioned out of the ICU and discharged the next day.
This botulism case report by Para et al. contrasts with the findings of an earlier chart-review study at Washington University, St Louis, MO, that was funded in part by Allergan and sought to determine the safety profile of high-dose (from 15 to 25 units/kg) onabotulinumtoxinA for lower-limb hypertonicity in children with cerebral palsy.[102] This study involved 929 onabotulinumtoxinA treatment encounters in 261 patients whose ages ranged from six months to 21 years and 32% of whom were non-ambulatory. The study authors reported that “[n]o patient developed botulism” during the study follow-up period (average of 6.5 weeks after treatment). They concluded that higher onabotulinumtoxinA doses are well tolerated over time and are safe in children with a spectrum of cerebral palsy phenotypes.
In the context of unapproved uses, Canadian physicians from the University of Saskatchewan reported a case involving a 31-year-old woman who was diagnosed with iatrogenic botulism following her second treatment session with 100 units of onabotulinumtoxinA injected into each of her palms and an additional 50 units injected into each of her axilla.[103] The injections had been administered two weeks earlier in a grid pattern by a physician with extensive experience and skill using onabotulinumtoxinA. The patient’s symptoms included fatigue, dry mouth, blurred vision, mild headache, and bilateral hand weakness. Her electrophysiologic studies were suggestive of a presynaptic neuromuscular junction localization. By nine months, her physical exam and electrophysiology were completely normal. No other adverse events were reported among other patients who used the specific batch of onabotulinumtoxinA used in this patient, according to the authors. Notably, this patient had tolerated an initial dose of 50 units of onabotulinumtoxinA injected into each axilla.
3.3.3. Evidence from case reports and case series regarding systemic iatrogenic botulism with low or recommended BT product doses
The following case reports and case series present examples of patients who developed systemic iatrogenic botulism following initial or subsequent treatment with low or recommended doses of approved indications of BT products.
In 1997, Bakheit et al. described two cases of adult females who developed generalized symptoms of a “syndrome which resembles botulism” after receiving intramuscular injections of abobotulinumtoxinA drawn from two different batches of the drug to treat localized muscle spasticity.[104] The total injected dose per session in both cases did not exceed 1,000 units, per the recommended dose of this BT product:
- The first patient, who had multiple sclerosis, developed right partial ptosis, dysphonia, flaccid severe paraplegia, and severe weakness of neck flexors after the first dose of abobotulinumtoxinA.
- The second patient, who had multisystem atrophy, developed diplopia, dysphagia, severe dysarthria, and moderately severe generalized weakness involving the neck, trunk, and limb muscles after her abobotulinumtoxinA injection, despite having tolerated the same dosage and injection frequency (every two to three months) for five years.
The authors also reported that there were EMG changes in all muscles tested among these patients. The muscle weakness and EMG changes resolved a few weeks later. The authors ruled out the possibility of dispensing errors or overdosing as potential causes of the systemic symptoms in both patients. Concluding their case report, they called for regular long-term monitoring of patients treated with BT therapy because the “absence of adverse effects at the commencement of treatment should not be relied on as evidence that a future injection would not cause an adverse response.”
Bhatia et al., 1999 reported three cases involving adult female patients with transient generalized weakness (two of them also had dysphagia) as well as consistent electrophysiological changes indicative of “mild botulism” after receiving recommended doses of abobotulinumtoxinA to treat dystonias (cervical dystonia in one patient and arm or leg dystonia in the other two).[105] All three patients developed their symptoms within two weeks after abobotulinumtoxinA injections, of which they had been receiving repeated therapeutic doses for the same indications, over the course of two and a half years in two patients and five years in the third. The weakness was temporally related to the abobotulinumtoxinA injections in these patients who all tested negative for acetylcholine receptor antibodies, did not take drugs known to impair neuromuscular transmission, and had no evidence of alternative causes of these symptoms, according to the authors. All three patients recovered from their botulism symptoms within three to six months. Whereas one of these patients did not receive further abobotulinumtoxinA injections, the other two did. Of those latter patients, one experienced a similar episode while the second did not have further problems with additional abobotulinumtoxinA injections. The authors recommended using BT treatment with caution in patients with symptomatic dystonia and other neurological disorders as they may be more prone to develop this complication. However, they cautioned that it is not clear which patients have a particularly high risk of developing mild botulism after BT treatment. Therefore, they advised clinicians to be aware that this complication can occur sporadically after injection of therapeutic doses in a patient who may have been receiving BT injections for many years without trouble.
Cobb et al., 2000 reported an incident that they characterized as the first known case of systemic botulism-like syndrome resulting in respiratory failure associated with BT therapy.[106] This case involved a 30-year-old female who presented to an emergency department after her second treatment session with 100 units of BT type A (mostly likely onabotulinumtoxinA) into the posterior neck muscles for cervical dystonia resulting from an automobile accident. She developed ptosis, catatonia, and collapsed approximately five hours after her BT injections. She then developed shallow respirations and loss of gag reflex and later developed respiratory arrest. The patient was intubated and mechanically ventilated. She experienced a complete recovery within 18 hours, before botulinum antitoxin was obtained from the CDC. The authors excluded the possibility of inadvertent intravascular administration of the BT product because a more rapid onset of symptoms would be expected in that case. They cautioned that clinicians should be aware that systemic effects may occur with localized BTA therapy and that these effects may be life-threatening.
Mezaki 2009 reported two cases of patients who developed “unexpected serious adverse reactions” to recommended doses of onabotulinumtoxinA:[107]
- The first case involved a 53-year-old woman who developed lagophthalmos and head instability for two months after an initial treatment with 90 units of onabotulinumtoxinA: 33 units for blepharospasm and 57 units for cervical dystonia. After the second treatment with 67 units of onabotulinumtoxinA in her periocular and cervical muscles, she developed difficulty in tongue protrusion and chewing as well as a transient choking sensation that lasted for two months.
- The second case involved a 76-year-old man with blepharospasm who developed immobilized eye lids and became unable to close his eyes voluntarily after receiving 16 units of onabotulinumtoxinA.
- The author speculated that certain individuals are “hypersensitive” to BT products, although this “seems uncommon.” Therefore, he recommended starting with the smallest required dose in BT-naive patients.
Coban et al., 2010 reported three cases involving female patients in their thirties who developed iatrogenic botulism symptoms after receiving 1,500 units of abobotulinumtoxinA to treat spasticity, although they had previously tolerated similar or lower therapeutic doses of this product:[108]
- Two of these patients had hereditary spastic paraparesis. The first developed dysphagia, respiratory distress, and generalized weakness three weeks after her second abobotulinumtoxinA session and was intubated to protect her airways after she developed aspiration pneumonia. The second patient developed dysphagia, dysarthria, shortness of breath, and weakness as well as mild weakness of the muscles of the neck, tongue, and upper extremity one week after her fourth abobotulinumtoxinA injection (previous injections were three to four months apart using the same protocol and dose schedule).
- The third patient had cerebral palsy (with no other disorders). One week after her third abobotulinumtoxinA treatment, she developed dysarthria, dysphagia, and moderately severe weakness in her neck, tongue, and trunk muscles.
All three patients received pyridostigmine (an off-label medication for botulism treatment) and recovered within one to six months. The authors noted that it is unlikely that the generalized muscle weakness in these patients was caused by an overdose of BT treatment, but more likely by a therapeutic dose of the product. They concluded that, because “botulism can occur sporadically after any therapeutic dose” of BT products, regular long-term monitoring is essential in patients treated with these products.
Ghasemi et al., 2012 described a case involving a 42-year-old woman with no history of neurological or systemic disorders or related risk factors, who developed iatrogenic botulism after she was injected with 30 units of onabotulinumtoxinA in her right axilla to treat primary axillary hyperhidrosis.[109] Notably, this dose is lower than the recommended one for this indication (50 unit per axilla). Two weeks later, she developed nasal speech, progressive dysphagia, and had noticeable weakness in her bulbar and upper limb muscles. The pattern of her clinical and related electrodiagnostic findings were consistent with “a typical prototype of subacute botulism,” according to the authors. After being counseled about other “serious symptoms, which might occur in the following days,” the patient was admitted four days later during a follow-up visit because she developed dyspnea, the authors added. The patient was given pyridostigmine for the next two months and recovered three months after her onabotulinumtoxinA injection. The authors concluded that the occurrence of “serious complications” despite using smaller-than-recommended doses of onabotulinumtoxinA means that further study of the optimal dosage of this drug for hyperhidrosis is needed.
Perot et al., 2015 described the case of a 39-year-old male with cerebral palsy who received repeated treatments with 200 units of onabotulinumtoxinA in each lower limb to treat spasticity.[110] He developed botulism-like symptoms (tiredness, muscle weakness, and deficit of shoulder and pelvic girdles) after his fourth treatment session. The clinical and EMG findings of this patient were suggestive of botulism-like syndrome, although the dose, dilution, and injection technique were the same as in his prior treatments, according to the authors. The author noted that the patient did not initially attribute his symptoms to BT therapy, suggesting the need for educating patients about these adverse effects after repeated BT injections.
Perhaps the case report by Halai et al., 2018 — coauthored by researchers from the CDC and the Los Angeles County Department of Public Health — represents the strongest case-study evidence regarding the occurrence of systemic iatrogenic botulism with recommended doses of BT products.[111] This case report provided a detailed account of a middle-aged woman who presented to a Los Angeles hospital emergency department with dysphagia and dysphonia without respiratory distress or weakness five days after receiving what the authors described as FDA-approved doses of BT type A product to treat cervical dystonia. The treatment was done under EMG guidance at a local medical office. This patient’s symptoms improved after she received HBAT, resulting in her discharge from the hospital the next day. Results of her laboratory testing (collected on the day of symptom onset and before HBAT administration) confirmed that she had iatrogenic botulism. The authors noted that subsequent investigations did not identify injection practice or dosing errors that had occurred with this patient.
Leonardi et al., 2019 reported two cases of iatrogenic botulism following therapeutic treatment with BT products, as supported by electrodiagnostic testing abnormalities:[112]
- The first case was a 48-year-old woman who initially received 400 units of onabotulinumtoxinA to treat lower limb spasticity associated with multiple sclerosis. She had a second treatment with onabotulinumtoxinA two months later, at a higher dosage of 600 units. Two weeks after her second injection, she complained of double vision, dysphonia, dysphagia, generalized weakness, and dry mouth. The patient had a suspected diagnosis of seronegative myasthenia gravis and was given pyridostigmine, which did not help with her symptoms. Five months later, she presented to the authors with general weakness and bulbar symptoms after her third treatment with 400 units of onabotulinumtoxinA. Her neurological examination showed multidirectional diplopia, bilateral ptosis, slow reactive pupils, accommodation deficit, dysphonia, dysphagia, and proximal tetraparesis. Her single-fiber EMG demonstrated an abnormally increased jitter with occurrence of blocks. Therefore, the authors diagnosed her with iatrogenic botulism based on her most recent onabotulinumtoxinA treatment. Her symptoms and impaired electrodiagnostic tests resolved without treatment in two months.
- The second case was a 32-year-old man with lower limb spasticity due to infantile cerebral palsy who was treated by the authors with a total dose of 1,500 units of abobotulinumtoxinA. Ten days later, he developed blurred vision, hoarse voice, swallowing difficulty, and generalized weakness. His neurological examination revealed impaired eye accommodation, multidirectional diplopia, bilateral facial weakness, and proximal tetraparesis. His single-fiber EMG showed abnormal jitter and blocks occurrence. His symptoms and neurophysiological abnormalities resolved fully without treatment in three months.
Iancu et al., 2020 reported a case involving a five-year-old female cerebral palsy patient with severe delay in her motor development — who had marked spasticity in her thigh adductor muscles and triceps, although she was able to sit, roll over, and stay in a hands and knees position but had difficulty alternating her legs.[113] She had tolerated an initial treatment with an unknown dose of BT treatment to treat her lower- and upper-limb spasticity. Four months later, she received a second BT injection of 1,000 units of abobotulinumtoxinA in her lower limbs only. Within a week of this second treatment, she was diagnosed with suspected iatrogenic botulism after experiencing eye and oropharyngeal effects (resulting in dysarthria, feeding troubles, swallowing difficulties, and right-side palpebral ptosis) as well as generalized weakness, and was subsequently hospitalized after developing pneumonia. Her single-fiber EMG showed increased jitter and synaptic latencies at neuromuscular junctions. Her symptoms slowly disappeared without treatment in approximately two months, and she did not have any subsequent BT injections. The authors recommended that, despite the rarity of “iatrogenic botulism” reports due to BT therapy, caregivers should be alerted to the possibility of this condition and that “careful” follow-up is necessary for patients receiving this treatment.
McGuinn et al., 2019 described a cosmetic case of a healthy 33-year-old woman who received 38 units of cosmetic onabotulinumtoxinA in her upper face (14 units in the glabella, 8 units in the forehead, and 16 units in the lateral orbital areas).[114] Four hours after this treatment session, the patient went for a six-mile run. Seven days later, she developed headache, difficulty swallowing, hoarseness, and right ear pain. Thirteen days after her treatment session, her dysphagia evaluation showed no oropharyngeal dysfunction, but she had mild weakness in her right trapezius. This patient gradually improved and recovered completely by the fourth week after her cosmetic onabotulinumtoxinA injection.
Unapproved uses
Although some of the case series in the previous section also included patients who developed systemic iatrogenic botulism or iatrogenic botulism-suggestive signs and symptoms after treatment with low-dose BT therapy for unapproved indications, we did not discuss such cases. There are other case reports in the literature documenting these adverse events in patients receiving BT products for unapproved indications. A related example in the therapeutic setting is that of a 30-year-old female patient who developed two episodes of generalized weakness and other botulism-like symptoms (including instability and difficulty with climbing stairs) after receiving 400 units of onabotulinumtoxinA in two sessions that were one year apart to treat palmoplantar hyperhidrosis, despite having tolerated an initial dose of this drug for the same indication earlier.[115] A related example in the cosmetic setting is a case report describing a 60-year-old female patient who developed mild to moderate dysphagia after receiving 60 units of abobotulinumtoxinA (equivalent to 20 units of onabotulinumtoxinA using a 3:1 conversion ratio) to smooth vertical platysma bands (an off-label indication), despite assertions of safe use of up to 100 units for this indication in previous patients.[116]
Recently, the European Centre for Disease Prevention and Control reported 67 cases of iatrogenic botulism resulting from intragastric BT therapy to treat obesity between February and March 2023.[117] Linked to a private Turkish hospital, most of these cases seem to have involved approved BT products. However, information about the BT doses used in these injections are not publicly available. Likewise, a U.S. case report linked the injection of 100 units of onabotulinumtoxinA in the pylorus to treat gastroparesis to systemic iatrogenic botulism.[118] Specifically, this patient — a 27-year-old woman with muscular dystrophy — developed weakness in her bilateral lower extremities that ascended to her upper extremities. She had received botulinum antitoxin approximately eight hours after her BT injection. After treatment in an intensive care unit, she was discharged to a rehabilitation center and eventually regained her baseline muscle strength six months after BT injection.
3.3.4. Evidence from pharmacovigilance studies
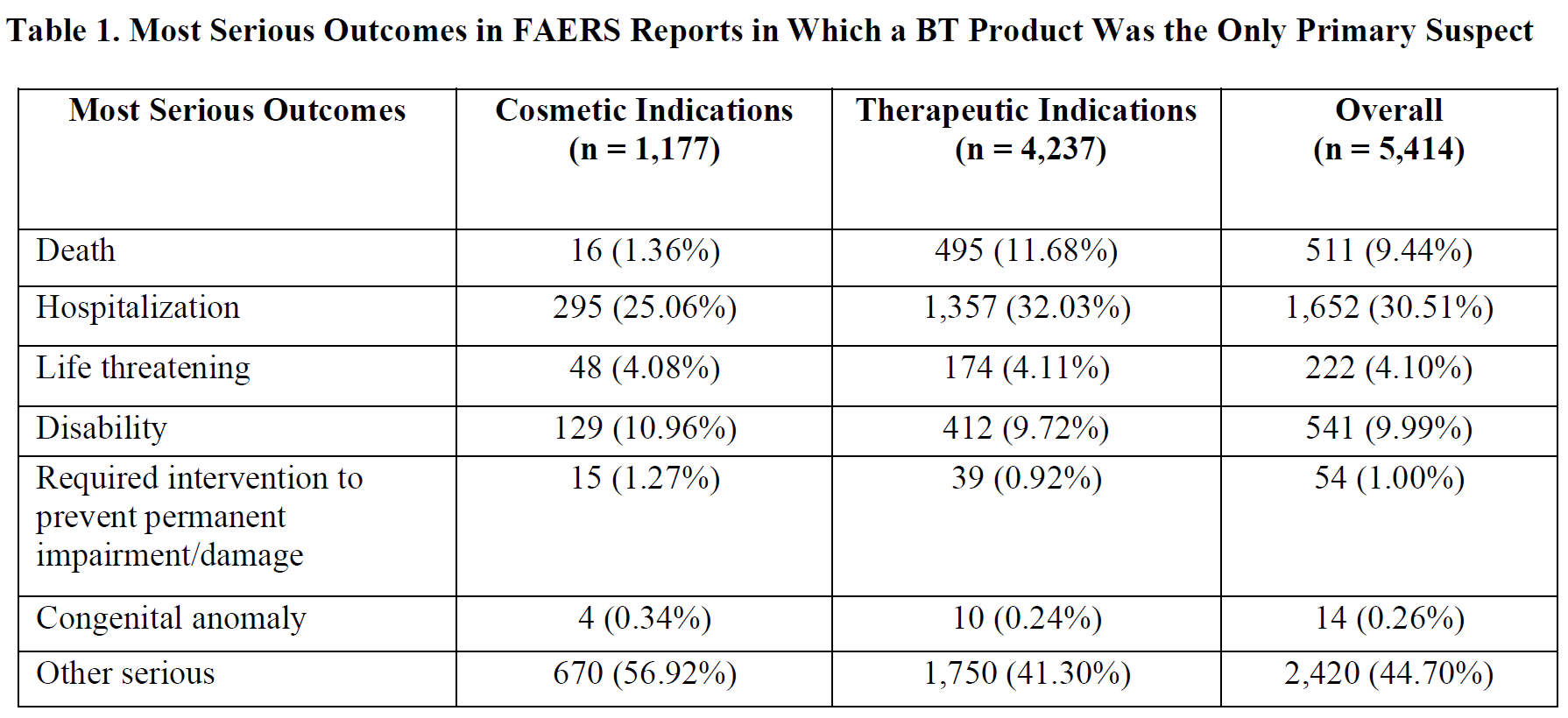
In 2005, FDA researchers Coté et al. raised safety issues about BT treatment in an analysis of FAERS data reported to the FDA between 1989 and 2003.[119] According to this analysis, there were 406 adverse event reports related to the therapeutic use of BT type A, which most likely involved only onabotulinumtoxinA given that the median reported therapeutic dose was 110 units. Of those, 217 experienced serious adverse events, and there were 28 deaths. The authors noted that most of the death cases had underlying comorbidities that could have contributed to the fatal outcome, making it hard to assess the role of BT treatment. There also were 36 serious reports related to cosmetic use of BT products. Of those reports, only six “did not display a pattern suggesting a common causal relationship” with BT products, according to the authors. Notably, the number of serious adverse events reported to Allergan increased with the concomitant increase in onabotulinumtoxinA sales, according to the authors.
Hristova et al., 2012 reported that they treated three patients who experienced fever-associated neurological adverse events after two to three treatment sessions with 50 to 300 units of a BT type A drug (most likely onabotulinumtoxinA) to treat focal dystonias.[120] This led them to analyze the FAERS database for reports pertaining to cosmetic and therapeutic BT therapy. Using a Freedom of Information request, the authors obtained deidentified BT product-related MedWatch reports from September 1992 to March 2009. Instead of conducting electronic searches for prespecified terms of symptoms, the authors appear to have read through each of the 3,087 individual reports they identified: 266 reports involved cosmetic indications and 2,821 involved therapeutic indications. Per their focus on nervous system adverse events, the authors determined that 36.5% and 24.3% of cosmetic and therapeutic BT-therapy reports, respectively, included nervous system-related adverse events. In addition, neurological adverse events were present in 40.6% of 123 reports that had a death outcome. They noted that “almost every other patient had disability, death, initial or prolonged hospitalization, a life-threatening event, or events requiring intervention to prevent permanent damage.” They also observed that adverse events happened in patients who received doses of as little as 12.5 units, suggesting that components of the BT-host interactions may not be dose related.
An important finding of the Hristova et al. analysis is that it identified 34 botulism cases, 58 cases with asthenia, 105 cases with cranial-nerve symptoms, and 63 with head paresthesia; none of these case reports included fever. Overall, 12 (10%) of the death cases involved asthenia, botulism, diplopia, gait problems, or weakness/paralysis. The authors proposed an algorithm for safer use of BT products until further research is conducted on their neurological safety, including:
- Transient symptoms including fever, headaches, nausea, and profound tiredness that occur after initial or repeated treatment sessions with BT products may be considered signs of a possible distant BT spread. Therefore, patients with these symptoms should be considered at a “high risk.”
- All patients — particularly those with mild symptoms and cosmetic users of BT products — should be warned that potential adverse events not related to the expected BT action may occur. Therefore, physicians should consider a benefit-risk assessment of these products when they offer them to patients with mild neurological or cosmetic impairment.
- Due to their complex biological effects and high potency, BT products may require a more vigilant and comprehensive reporting system.
Phadke et al., 2015 analyzed pharmacovigilance data from Health Canada and other major electronic databases regarding the use of onabotulinumtoxinA and incobotulinumtoxinA for focal spasticity from 2009 to 2013.[121] Overall, they identified 285 reports. Of these, 272 pertained to onabotulinumtoxinA (which was approved for lower- and upper-limb spasticity in Canada in 2001) while 13 pertained to incobotulinumtoxinA (which was approved for upper-limb spasticity in Canada in 2009). Fifty-three percent of the identified onabotulinumtoxinA reports were serious. Per the classification of the researchers, these adverse events were muscle weakness (15%), oropharyngeal related (15%), respiratory related (38%), eye related (23%), bowel or bladder related (15%), and infection related (15%). Similarly, the researchers found that commonly reported adverse events for BT products in the literature were muscle weakness, pain, as well as oropharyngeal, bowel/bladder, blood circulation, neurological, gait, and respiratory problems. The authors speculated that high doses of BT therapy may be implicated in adverse events, including muscle weakness. However, only 33% of 125 onabotulinumtoxinA users for whom the dose was reported had received greater than the recommended maximal cumulative dose for this drug. Although the authors — most of whom disclosed receiving previous funding from BLA holders of BT products — did consider the possibility that some of the serious adverse events may have been due to BT therapy, they noted that the presence of comorbidities also could partly explain some of the serious adverse events. In addition, the Health Canada data did not provide reasons for death and hospitalization, which occurred in 8% and 18% of the overall reports, respectively. There was no indication from these data whether there was a causal relationship between BT therapy and the adverse events associated with their use, according to the authors.
Montastruc et al., 2017 analyzed the World Health Organization’s VigiBase — a global database of individual case safety reports — for reports associated with the use of BT type A products.[122] For children (under 17 years), the authors identified 162 reports from January 1995 to August 2015 in which a BT type A product was used to treat spasticity or cerebral palsy. Of those, 67% were serious reports. This meant that they had an outcome of death, life-threatening events, triggered hospitalization (or prolongation of existing hospitalization), led to persistent incapacity or disability, or were otherwise determined to be “clinically relevant” by the physician who reported the case. Death was reported in 12% of all 162 reports pertaining to children. The most common adverse events in these reports were dysphagia or aspiration (17%), asthenia or fatigue (16%), muscular weakness (16%), and pneumonia (10%). Importantly, botulism was reported in 7% of these reports. The authors noted that 83% of all 162 reports were reported after the boxed warning was added in 2008.
For adults, there were 344 reports from 1991 to 2015 in which a BT product was used for spasticity. Death was reported in 8% of these reports. The most common adverse events in these reports were asthenia or fatigue (20%), muscular weakness (17%), dysphagia or aspiration (17%), dyspnea (10%), and pneumonia (5%). Additionally, botulism was reported in 4% of these reports.
The authors noted that the identified adverse reaction reports — which mainly appear to be linked to a systemic spread of BT products — were “quite similar” between children with cerebral palsy and adults. However, proportionality analyses conducted by the authors showed more statistically significant deaths in children than in adults due to exposure to BT type A used to treat spasticity. The authors could not adjust for preexisting medical comorbidities of children because that information was not available in VigiBase. In an accompanying commentary to this analysis, Yiannakopoulou noted that the risk-benefit ratio of BT type A products can be evaluated before therapy in all therapeutic or cosmetic uses of these products, not just in patients with cerebral palsy.[123] Therefore, physicians must establish long-term follow-up of patients treated with BT products and need to inform them about the risk of serious and even fatal adverse events associated with the use of these products.
Ahsanuddin et al., 2021 analyzed the FAERS database for the top adverse events reports for abobotulinumtoxinA, incobotulinumtoxinA, and onabotulinumtoxinA, excluding reports with off-label uses.[124] Although they did not specify a timeframe for their study, it was likely inclusive of all related reports in the database around July 2020, the date of their article submission. To validate their results and examine the clinical significance of the top adverse events, the authors used two measures of disproportionality analysis: proportional reporting ratios (PRRs) and reporting odds ratios (RORs).
Overall, the authors identified 46 cases of botulism, 428 reports of dysphagia, and 85 cases of death for evaluated BT products. For botulism, the PRR and ROR signal scores reflected a statistically significant association with both abobotulinumtoxinA and onabotulinumtoxinA, suggesting that “botulism raise[s] a concern for patient safety,” compared with reports for all other drugs in the database. For dysphagia, the PRR and ROR signal scores reflected a statistically significant association for abobotulinumtoxinA only. In contrast, neither the PRR nor ROP signal scores for death reflected a statistical association for any BT product.
Overall, there were 439 cases of muscular weakness and 355 cases with asthenia associated with BT products in Ahsanuddin et al.’s analysis. Whereas the PRR and ROR signal scores for muscle weakness reflected a statistically significant association for abobotulinumtoxinA only, the corresponding signal scores for asthenia did not reflect a statistically significant association for any BT product. Additionally, the PRR and ROR signal scores for each of eyelid ptosis (occurring in 313 reports), eyebrow ptosis (occurring in 23 reports), and facial paresis (occurring in 106 reports) reflected a statistically significant association for both of abobotulinumtoxinA and onabotulinumtoxinA. In conclusion, the authors suggested that signing a consent form listing these adverse events, in addition to botulism, may be recommended for consumers at the time of treatment.
Zargaran et al., 2022 analyzed adverse events of BT type A products that had facial cosmetic indications in a pharmacovigilance registry of the UK’s Medicines and Healthcare products Regulatory Agency.[125] Overall, there were only 188 reports involving cosmetic indications for BT type A products in this registry from 1991 to 2020, likely reflecting underreporting, as suggested by the authors. Of those, 47.3%, 38.3%, and 8.5% pertained to the use of onabotulinumtoxinA, abobotulinumtoxinA, and incobotulinumtoxinA, respectively. Three of these facial cosmetic reports included botulism as an adverse event. Other commonly reported adverse events included general (including asthenia, fatigue, fever, and influenza-like symptoms) adverse events (28.2%), dysesthesia (18.6%), musculoskeletal symptoms (18.6%), facial paresis or paralysis (14.4%), and dysphagia (9.0%). Unfortunately, no information was provided by the authors about the dosage of BT products in the reports that they analyzed. Nonetheless, in all 188 reports with facial cosmetic BT type A indications, 35% of patients recovered completely while 36% of patients had residual symptoms and were “without full recovery.” In addition to concluding that adverse events for facial cosmetic indications are likely insufficiently reported in the UK, the authors cautioned that reported cases were “often complex with multiple adverse drug reactions.”
3.3.5. Evidence from our FAERS analysis
Report selection criteria
We analyzed the FAERS database from January 1998 to March 2021 for all reports with one or more serious outcome(s) in which any approved BT product was listed as the only “primary suspect” drug. For each of these reports, we identified the single most serious outcome based on the following hierarchical categories: (1) death, (2) life-threatening, (3) hospitalization — initial or prolonged, (4) disability or permanent damage (hereafter, disability), (5) congenital anomaly, (6) required intervention to prevent permanent impairment/damage (hereafter, required intervention), and (7) other serious (important medical event). We excluded reports with multiple BT products as well as those with preferred terms indicating accidental or inadvertent administration, injection errors, and overdose. However, we did not exclude reports of doses that exceed the maximum doses for respective BT products. We identified cosmetic reports with “skin cosmetic procedure” or “skin wrinkling” as the only indication. We classified reports that specified “Botox Cosmetic” as the primary suspect drug as a “cosmetic report” even if no indications were listed.
Overall serious reports
Of the overall 5,414 reports, 1,177 (21.74%) involved cosmetic indications and the remaining 4,237 (78.26%) reports involved therapeutic indications. As shown in Table 1, after other serious outcomes, hospitalization was the most frequent serious outcome, occurring in 30.51% of the overall reports, followed by disability (9.99%) and death (9.44%).
Overall, 3,412 (63.02%) of these reports did not include any reported concomitant medications. Based on the FDA report date variable, 3,851 (71.13%) of the overall reports were received by the FDA within the last 13 years, i.e., after the FDA’s boxed-warning requirement in 2009 for BT products.
Frequencies of BT treatment
Using the dose and related frequency variables in the serious BT-related reports, we tried to identify reports indicating initial or single versus repeated doses for each specified BT product. As shown in Table 2, we determined that 580 (10.71%) of the overall reports pertained to repeated injections of BT products, some occurring on pro re nata (PRN) or periodic (e.g., every 3 or more months) bases, with over a dozen reports indicating that the adverse effects occurred several years after the use of BT products. Meanwhile, 3,008 (55.56%) reports appear to pertain to initial or single-dose injections. Of the remaining reports, 1,826 (33.73%) either did not specify the frequency of BT product injection or did not report any dose or frequency information (hereafter, missing).
Reports with iatrogenic botulism-suggestive signs and symptoms
Like the FDA’s 2009 FAERS analysis,[126] we tried to identify serious BT-related case reports with signs and symptoms that are suggestive of iatrogenic botulism, consistent with the pharmacologic effect of BT products. We determined these signs and symptoms based on the work of Phadke et al. and of CDC researchers.[127],[128] We selected these signs and symptoms based on our review of the adverse reactions listed in our FAERS analytic sample and grouped them into six categories: bladder-related, muscle weakness or paralysis (in the extremities), ocular, oropharyngeal, respiratory, and sensory-related (limited to paranesthesia, peripheral sensory neuropathy, and hypoesthesia) (see Table 3).
Of the overall 5,414 BT-related serious reports, 2,817 (52.03%) listed signs and symptoms pertaining to at least one iatrogenic botulism-suggestive category (Table 3). Of those, 2,138 (75.90%) involved therapeutic indications and 679 (24.10%) involved cosmetic indications. Most of the overall reports with signs and symptoms pertaining to at least one of the iatrogenic botulism-suggestive categories involved therapeutic onabotulinumtoxinA (n = 1,621), cosmetic onabotulinumtoxinA (n = 554), and abobotulinumtoxinA (n = 439). Fewer reports pertained to rimabotulinumtoxinB (n = 118), incobotulinumtoxinA (n = 76), and prabotulinumtoxinA-xvfs (n = 9). Signs and symptoms pertaining to each of the muscle weakness or paralysis, oropharyngeal, and respiratory categories were reported in comparable proportions (ranging from 18.08% to 27.87%) of cosmetic and therapeutic reports. In contrast, signs and symptoms pertaining to the ocular and sensory-related categories were reported more frequently in cosmetic than therapeutic reports. Death, disability, and hospitalization were the most serious outcomes, reported in 5.36%, 13.17%, and 31.27% of the 2,817 reports with signs and symptoms that are suggestive of iatrogenic botulism, respectively. Additionally, of those latter reports, 1,868 (66.31%) were reported to the FDA after the boxed-warning requirement. Importantly, 59.71% of these reports did not indicate the use of any concomitant medications.
In terms of BT treatment frequency, 1,570 (55.73%) of the 2,817 reports with signs and symptoms that are suggestive of iatrogenic botulism pertained to initial or single doses and 269 (9.55%) reports pertained to repeated doses. Of the latter reports, 215 reports involved therapeutic indications and 54 reports involved cosmetic indications. The remaining 978 (34.71%) reports had missing dosing frequency information. Overall, many of the reports with various signs and symptoms that are suggestive of iatrogenic botulism showed that low BT product doses were injected. For example, for cosmetic onabotulinumtoxinA reports, there were over 140 initial or single dose reports and at least 24 repeated-injection reports that indicated that 30 units or fewer doses of this drug were injected (some were as low at 6 units).
Iatrogenic botulism reports
Of the overall 5,414 BT-related serious case reports in our analysis, 121 (2.23%) specified botulism as an adverse effect: 89 involved therapeutic indications and 32 involved cosmetic indications. Most of these reports pertained to onabotulinumtoxinA (n = 66) and cosmetic onabotulinumtoxinA (n = 27). AbobotulinumtoxinA and incobotulinumtoxinA were the only primary drug suspects in 14 and 13 reports, respectively. RimabotulinumtoxinB was the only primary drug suspect in one report. Importantly, 90 (74.38%) of the 121 botulism reports listed signs and symptoms pertaining to at least one of the botulism-suggestive categories described in Table 3. Notably, of the botulism reports involving cosmetic onabotulinumtoxinA, 25 (92.59%) listed signs and symptoms pertaining to at least one of the botulism-suggestive categories; 20 (74.07%) reports listed signs and symptoms pertaining to two or more of these categories. Importantly, 74.38% of the overall 121 botulism reports did not report use of any concomitant medications (the proportions for each of the cosmetic and therapeutic indications were 71.88% and 75.28%, respectively).
Of the overall 121 botulism reports, 100 (82.64%) were reported to the FDA after the boxed-warning requirement. Whereas hospitalization and other serious outcomes were the most common outcomes reported in 47.93% and 34.71% of the overall botulism reports, respectively, death was reported in 3.31% of these reports, and disability and life-threatening outcomes were reported in 4.13% and 9.92% of them, respectively. Even though only two of the overall 121 botulism reports listed cerebral palsy as the drug indication, eight other therapeutic BT-product reports involved children. Some of those eight reports listed muscle spasticity as the drug indication. Other therapeutic indications in the remaining botulism cases were blepharospasm, migraine, torticollis, dystonia, hyperhidrosis, certain off-label uses (e.g., autism or depression), or unspecified indications.
BT products were listed as an initial or single-dose treatment in 86 (71.07%) of the botulism reports, as a repeated dose in 3 (2.48%) reports, and the treatment frequency was missing for the remaining reports. We found that several of the botulism cases involved low doses of BT products. For example, seven of the 27 reports involving cosmetic onabotulinumtoxinA reported that the injected doses of this product ranged from 10 to 30 units. In addition, five of the 66 reports involving therapeutic uses of onabotulinumtoxinA reported injected doses of 50 or fewer units.
Reports with dechallenge or rechallenge information
As is typical with the FAERS data, the medication dechallenge and rechallenge information was sparsely populated in our analytic sample. As shown in Table 4, positive dechallenge (in which patients experienced an improvement in adverse events after discontinuation of the specified BT product) was reported in 132 (2.44%) of the overall 5,414 serious case reports associated with BT products. Most of these reports involved therapeutic onabotulinumtoxinA (n = 94) and cosmetic onabotulinumtoxinA (n = 24). Of the total positive dechallenge reports, 75 (56.82%) included signs and symptoms pertaining to at least one botulism-suggestive category and two reports (1.52%) included botulism as an adverse effect. Many of these reports included recommended BT product doses for the listed indications (including blepharospasm, dystonia, migraine, muscle spasticity, and skin wrinkling, etc.).
Positive rechallenge (in which adverse reactions recurred after the reintroduction of the specified BT product) was reported in 56 (1.03%) reports. Most of these reports involved onabotulinumtoxinA (n = 33) and cosmetic onabotulinumtoxinA (n = 17). Of the total positive-rechallenge reports, 36 (64.29%) included signs and symptoms pertaining to at least one botulism-suggestive category. Several of these reports included recommended BT product doses for the listed indications (including blepharospasm, dystonia, migraine, muscle spasticity, and skin wrinkling, etc.). Finally, both positive dechallenge and rechallenge were reported in only 22 (0.41%) reports; 18 reports involved therapeutic onabotulinumtoxinA and four reports involved cosmetic onabotulinumtoxinA. Of those 22 reports, 13 (59.09%) included signs and symptoms pertaining to at least one botulism-suggestive category. Nearly half of the 22 positive dechallenge and rechallenge reports included recommended BT product doses for the listed indications. Most (79.55%) of the reports with dechallenge information and 55.36% of those with rechallenge information pertained to reports that preceded the boxed-warning requirement.
Key limitations of our FAERS analysis
Although we identified over 5,400 serious FAERS reports in which a single BT product was the only reported primary suspect, there are several limitations to this analysis. Most likely, this analysis greatly underestimates the true incidence of serious adverse events associated with BT therapy — including those of iatrogenic botulism and its suggestive signs and symptoms — due to the voluntary reporting nature of these events. For example, a systematic review of studies from the U.S. and other developed countries estimated that only 6% of adverse events are reported to spontaneous reporting systems.[130] Other studies show similar reporting trends. The extent of underreporting for BT products is likely even higher, as one study showed that, although around 2,000 BT therapy sessions were carried out yearly for several years in a French rehabilitation medicine unit and physicians noted adverse events in medical files, adverse events for only four of these injections were reported.[131] In addition, the CDC indicates that some botulism cases may not be recognized either because of misdiagnosis or because mildly affected patients may not seek medical care.[132] This is particularly relevant in the case of iatrogenic botulism because it can occur up to weeks after BT therapy. Additional limitations are that the documentation of adverse events in these reports does not necessarily mean that they are causally related to the reported suspect products and instead may be related to underlying diseases or other concomitant medications and, finally, there are likely sizeable missing data, particularly regarding the BT dosing information.
3.3.6. Discussion of presented evidence and conclusions
The collective evidence from case reports, case series (including one CDC case report),[133] pharmacovigilance studies, and our FAERS analysis demonstrates an association between treatment with initial or repeated recommended doses of BT products and systemic iatrogenic botulism that may necessitate timely administration of botulinum antitoxin. Although this postmarketing evidence cannot definitively establish a cause-and-effect relationship, it meets some of the factors that constitute “reasonable evidence of a causal association” of a clinically significant hazard with a drug, as listed in section 1.1 of this petition.[134] First, the occurrence of systemic iatrogenic botulism at recommended doses of BT products has both biological and theoretical plausibility. This is because the therapeutic effect of BT products occurs at the neuromuscular junction, which is the same area where systemic iatrogenic botulism occurs. In essence, systemic signs and symptoms of iatrogenic botulism are an exaggeration of the therapeutic effect of BT products. Therefore, the notion that iatrogenic botulism can occur with recommended doses of BT products, even if previous doses were tolerated, is biologically plausible. Second, the presented evidence from case reports, case series, and our FAERS analysis supports a temporal association between BT therapy and systemic iatrogenic botulism and iatrogenic botulism-suggestive signs and symptoms. Particularly, the authors of the case reports and case series discussed in our petition emphasized the lack of alternative explanations for systemic iatrogenic botulism and its suggestive signs and symptoms. Additionally, in our FAERS analysis, BT products were the only primary suspect drugs in these reports. Most reports with systemic iatrogenic botulism and its suggestive signs and symptoms associated with the use of BT products — including those involving recommended BT doses — did not report use of concomitant medications. Finally, although the dechallenge and rechallenge information was limited, it was positive in large proportions of the small number of reports with this information, including those involving recommended BT doses.
We cannot rule out that underlying conditions could have caused systemic iatrogenic botulism and its suggestive signs and symptoms in the BT-treated case reports and pharmacovigilance evidence described in this petition. However, the FDA stated in 2009, “a potential association between use of [BT] and symptoms consistent with iatrogenic botulism could not be excluded.”[135] Similarly, we argue that a potential association between recommended BT doses and iatrogenic botulism cannot be ruled out.
Therefore, we urge the FDA to exercise its regulatory authority to require the modification of the boxed warning of BT products to make it clear that reports of severe systemic iatrogenic botulism or iatrogenic botulism-suggestive signs and symptoms — that have required hospitalization and treatment with botulinum antitoxin — have occurred with initial and repeated recommended doses of these products. Even though the incidence of this risk appears to be low, it should be communicated clearly in the boxed warning of these products. Updating the boxed warning of BT products is necessary for public health, given the widespread use of these products, the increasing list of approved and unapproved indications, and the often-repeated use of these products by consumers who may think that the risk of systemic iatrogenic botulism is limited to their use in high doses or to their use in individuals with neurological conditions.
4. Requested Action 2: Remove misleading promotional claims from product labeling
4.1. Action 2 and its rationales
Remove the following misleading promotional paragraph (a) from the labeling of onabotulinumtoxinA (Botox), paragraph (b) from the labeling of onabotulinumtoxinA (Botox Cosmetic), and paragraph (c) from the medication guide of both Botox and Botox Cosmetic:
(a) “No definitive serious adverse event reports of distant spread of toxin effect associated with BOTOX for blepharospasm at the recommended dose (30 Units and below), severe primary axillary hyperhidrosis at the recommended dose (100 Units), strabismus, or for chronic migraine at the labeled doses have been reported.”
(b) “No definitive serious adverse event reports of distant spread of toxin effect associated with dermatologic use of BOTOX /BOTOX Cosmetic at the labeled dose of 20 Units (for glabellar lines), 24 Units (for lateral canthal lines [crow’s feet]), 40 Units (for forehead lines with glabellar lines), 44 Units (for simultaneous treatment of lateral canthal lines and glabellar lines), 64 Units (for simultaneous treatment of lateral canthal lines, glabellar lines, and forehead lines), or 100 Units (for severe primary axillary hyperhidrosis) have been reported.”
(c) “There has not been a confirmed serious case of spread of toxin effect away from the injection site when BOTOX has been used at the recommended dose to treat chronic migraine, severe underarm sweating, blepharospasm, or strabismus, or when BOTOX Cosmetic has been used at the recommended dose to treat frown lines, crow’s feet lines, and/or forehead lines.”
The above claims are problematic for two main reasons. First, the blanket claims regarding a lack of cases involving “definitive” or “confirmed” distant spread of onabotulinumtoxinA are refutable. For example, from the serious FAERS reports associated with approved BT products described under section 3.3.5 of this petition, we identified 639 onabotulinumtoxinA reports in which one of the following four indications was the only one reported: blepharospasm, hyperhidrosis, migraine (or headache), or strabismus. Of those, 269 (42.10%) reports included signs and symptoms pertaining to at least one of the iatrogenic botulism-suggestive categories described in Table 3. Importantly, these signs and symptoms were reported in numerous reports involving low and recommended initial or repeated onabotulinumtoxinA doses for each indication. As discussed earlier, of the 934 reports involving cosmetic onabotulinumtoxinA, there were over 140 initial or single-dose reports and at least 24 repeated-dose reports with 30 units or lower (some were as low as 6 units) of this product. It is implausible for Allergan (or its now parent company AbbVie) to argue that none of these case reports are definitive or confirmatory of distant spread of onabotulinumtoxinA. Second, these claims are not present in similar onabotulinumtoxinA labeling in other countries, such as Canada and the UK.[136],[137],[138]
As described under the related legal standard in section 1.2. of this petition, it is incumbent on the FDA to require the removal of these misleading claims because they only serve to circumvent the boxed warning and render the labeling of cosmetic onabotulinumtoxinA (Botox, Botox Cosmetic) misbranded, per the FD&C Act. Failing to do so would mislead clinicians and patients into discounting the boxed warning of these products and possibly other approved BT products as well.
5. Conclusion
In 2009 — 20 years after its initial approval of the first BT product — the FDA correctly began requiring a boxed warning regarding the risk of distant spread of BT products, based on the FAERS analysis in our previous petition[139] and the agency’s own analysis,[140] along with other related evidence.
Today, 14 years later, we urge the agency to respond to the postmarketing evidence presented in this petition and update the boxed warning of BT products to make the risk of their distant spread clearer. The updated warning should specify that reports of severe systemic iatrogenic botulism or iatrogenic botulism-suggestive signs and symptoms, that may require the administration of botulinum antitoxin, have occurred with both initial and repeated use of recommended doses of approved BT products for various indications. We also urge the agency to remove from the labeling of onabotulinumtoxinA (Botox, Botox Cosmetic) promotional claims that mislead the public and circumvent the boxed warning.
Specifically, we hereby petition the FDA under the FD&C Act (21 U.S.C. § 352) and under FDA regulations at 21 C.F.R. § 10.30 and § 201.56 to promptly require the following actions:
(1) Modify the existing boxed warning in the product labeling of abobotulinumtoxinA (Dysport), daxibotulinumtoxinA-lanm (Daxxify), incobotulinumtoxinA (Xeomin), onabotulinumtoxinA (Botox, Botox Cosmetic), prabotulinumtoxinA-xvfs (Jeuveau), and rimabotulinumtoxinB (Myobloc) to emphasize the risk of severe systemic iatrogenic botulism. We do not distinguish between various approved BT products in this regard because the risk applies to all of them. We suggest the following language to enhance the current boxed warning:
Postmarketing cases of severe systemic iatrogenic botulism and related symptoms — many of which have required hospitalization and treatment with botulinum antitoxin — have occurred within hours to weeks after injection of botulinum-toxin products, including those intended for cosmetic uses. These cases occurred even with recommended doses in patients who had an initial injection as well as in those who had tolerated previous injections of these products. Signs and symptoms of iatrogenic botulism may include asthenia, generalized muscle weakness, diplopia, ptosis, dysphagia, dysphonia, dysarthria, urinary incontinence, and breathing difficulties. Swallowing and breathing difficulties can be life threatening, and there have been reports of death. Therefore, caution should be exercised upon each administration of BT products. Particularly, monitoring and prompt recognition of impending systemic iatrogenic botulism as well as early administration of botulinum antitoxin can be critical to improving patient outcomes associated with these potentially life-threatening adverse events.
Conforming changes also should be made in other sections of the labeling of BT products.
(2) Remove the following misleading promotional paragraph (a) from the labeling of onabotulinumtoxinA (Botox), paragraph (b) from the labeling of onabotulinumtoxinA (Botox Cosmetic), and paragraph (c) from the medication guide of both Botox and Botox Cosmetic:
(a) “No definitive serious adverse event reports of distant spread of toxin effect associated with BOTOX for blepharospasm at the recommended dose (30 Units and below), severe primary axillary hyperhidrosis at the recommended dose (100 Units), strabismus, or for chronic migraine at the labeled doses have been reported.”
(b) “No definitive serious adverse event reports of distant spread of toxin effect associated with dermatologic use of BOTOX /BOTOX Cosmetic at the labeled dose of 20 Units (for glabellar lines), 24 Units (for lateral canthal lines [crow’s feet]), 40 Units (for forehead lines with glabellar lines), 44 Units (for simultaneous treatment of lateral canthal lines and glabellar lines), 64 Units (for simultaneous treatment of lateral canthal lines, glabellar lines, and forehead lines), or 100 Units (for severe primary axillary hyperhidrosis) have been reported.”
(c) “There has not been a confirmed serious case of spread of toxin effect away from the injection site when BOTOX has been used at the recommended dose to treat chronic migraine, severe underarm sweating, blepharospasm, or strabismus, or when BOTOX Cosmetic has been used at the recommended dose to treat frown lines, crow’s feet lines, and/or forehead lines.”
C. ENVIRONMENTAL IMPACT
We claim categorical exclusion under 21 C.F.R. § 25.31(a) from the environmental assessment requirement. An assessment is not required because the requested action would not increase the use of the active moiety that is the subject of this petition.
D. ECONOMIC IMPACT
Will be submitted upon request.
E. CERTIFICATIONS
We certify that, to the best of our knowledge and belief, this petition includes all information and views on which this petition relies, and that it includes representative data and information known to the petitioners which are unfavorable to the petition.
Azza AbuDagga, M.H.A., Ph.D.
Health Services Researcher
Public Citizen’s Health Research Group
1600 20th Street, N.W.
Washington, DC 20009
202-588-1000
Sidney M. Wolfe, M.D.
Founder and Senior Adviser
Public Citizen’s Health Research Group
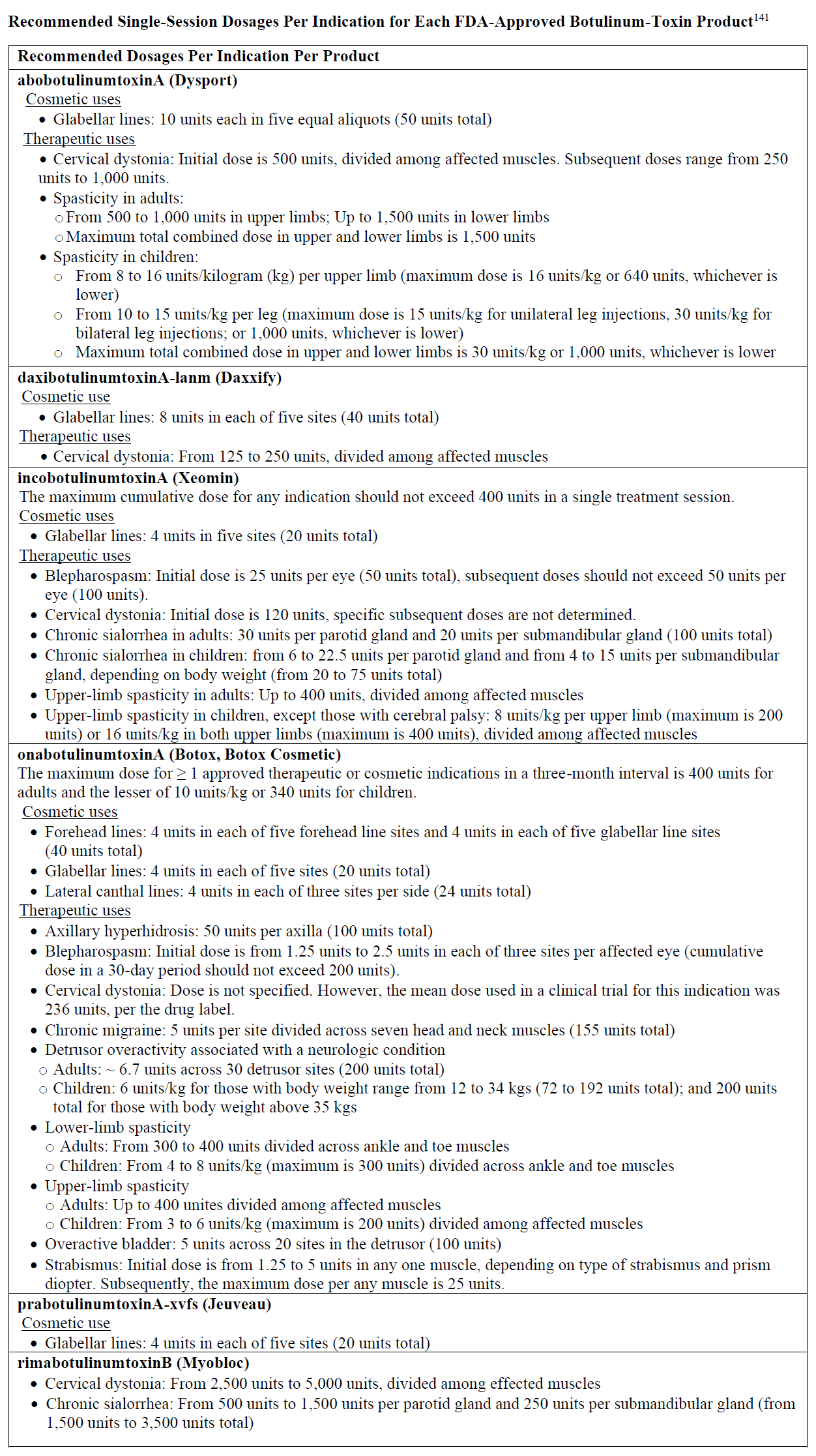
[141] Specific dosages for children are noted, where applicable/available.
References
[1] Ipsen Biopharmaceuticals, Inc. Label: abobotulinumtoxinA (DYSPORT). September 2023. https://www.accessdata.fda.gov/drugsatfda_docs/label/2023/125274s125lbl.pdf. Accessed November 13, 2023.
[2] Revance Therapeutics, Inc. Label: daxibotulinumtoxinA-lanm (DAXXIFY). August 2023. https://www.accessdata.fda.gov/drugsatfda_docs/label/2023/761127s002lbl.pdf. Accessed November 13, 2023.
[3] Merz Pharmaceuticals, Inc. Label: incobotulinumtoxinA (XEOMIN). September 2023. https://www.accessdata.fda.gov/drugsatfda_docs/label/2023/125360s097lbl.pdf. Accessed November 13, 2023.
[4] Allergan, Inc. Label: onabotulinumtoxinA (BOTOX). August 2023. https://www.accessdata.fda.gov/drugsatfda_docs/label/2023/103000Orig1s5325Correctedlbl.pdf. Accessed November 13, 2023.
[5] Allergan, Inc. Label: onabotulinumtoxinA (BOTOX COSMETIC). February 2021. https://dailymed.nlm.nih.gov/dailymed/fda/fdaDrugXsl.cfm?setid=485d9b71-6881-42c5-a620-a4360c7192ab&type=display. Accessed November 13, 2023.
[6] Evolus Inc. Label: prabotulinumtoxinA-xvfs (JEUVEAU). April 2021. https://dailymed.nlm.nih.gov/dailymed/fda/fdaDrugXsl.cfm?setid=17a914c1-e54b-4b50-965d-b0fd9111bba4&type=display. Accessed November 13, 2023.
[7] Solstice Neurosciences, LLC. Label: rimabotulinumtoxinB (MYOBLOC). March 2021. https://dailymed.nlm.nih.gov/dailymed/fda/fdaDrugXsl.cfm?setid=675cb354-9d13-482e-8ac2-22f709c58b4f&type=display. Accessed November 13, 2023.
[8] Allergan, Inc. Label: onabotulinumtoxinA (BOTOX). August 2023. https://www.accessdata.fda.gov/drugsatfda_docs/label/2023/103000Orig1s5325Correctedlbl.pdf. Accessed November 13, 2023.
[9] Allergan, Inc. Label: onabotulinumtoxinA (BOTOX COSMETIC). February 2021. https://dailymed.nlm.nih.gov/dailymed/fda/fdaDrugXsl.cfm?setid=485d9b71-6881-42c5-a620-a4360c7192ab&type=display. Accessed November 13, 2023.
[10] 21 C.F.R. § 201.57.
[11] Food and Drug Administration. Guidance for industry: Warnings and precautions, contraindications, and boxed warning sections of labeling for human prescription drug and biological products — content and format. October 2011. https://www.fda.gov/media/71866/download. Accessed November 13, 2023.
[12] 21 C.F.R. § 201.57.
[13] Food and Drug Administration. Guidance for industry: Warnings and precautions, contraindications, and boxed warning sections of labeling for human prescription drug and biological products — content and format. October 2011. https://www.fda.gov/media/71866/download. Accessed November 13, 2023.
[14] 21 U.S.C. § 352(a)(1).
[15] 21 C.F.R. § 201.56(a)(2).
[16] Rao AK, Sobel J, Chatham-Stephens K, et al. Clinical guidelines for diagnosis and treatment of botulism, 2021. MMWR Recomm Rep. 2021;70(2):1-30.
[17] Allergan, Inc. Label: onabotulinumtoxinA (BOTOX). August 2023. https://www.accessdata.fda.gov/drugsatfda_docs/label/2023/103000Orig1s5325Correctedlbl.pdf. Accessed November 13, 2023.
[18] Apkon SD, Cassidy D. Safety considerations in the use of botulinum toxins in children with cerebral palsy. PM R. 2010;2(4):282-284.
[19] Bottrill K. Growing old disgracefully: The cosmetic use of botulinum toxin. Altern Lab Anim. 2003;31(4):381-391.
[20] Apkon SD, Cassidy D. Safety considerations in the use of botulinum toxins in children with cerebral palsy. PM R. 2010;2(4):282-284.
[21] Allergan, Inc. Label: onabotulinumtoxinA (BOTOX). August 2023. https://www.accessdata.fda.gov/drugsatfda_docs/label/2023/103000Orig1s5325Correctedlbl.pdf. Accessed November 13, 2023.
[22] Allergan, Inc. Label: onabotulinumtoxinA (BOTOX COSMETIC). February 2021. https://dailymed.nlm.nih.gov/dailymed/fda/fdaDrugXsl.cfm?setid=485d9b71-6881-42c5-a620-a4360c7192ab&type=display. Accessed November 13, 2023.
[23] Ipsen Biopharmaceuticals, Inc. Label: abobotulinumtoxinA (DYSPORT). September 2023. https://www.accessdata.fda.gov/drugsatfda_docs/label/2023/125274s125lbl.pdf. Accessed November 13, 2023.
[24] Merz Pharmaceuticals, Inc. Label: incobotulinumtoxinA (XEOMIN). September 2023. https://www.accessdata.fda.gov/drugsatfda_docs/label/2023/125360s097lbl.pdf. Accessed November 13, 2023.
[25] Solstice Neurosciences, LLC. Label: rimabotulinumtoxinB (MYOBLOC). March 2021. https://dailymed.nlm.nih.gov/dailymed/fda/fdaDrugXsl.cfm?setid=675cb354-9d13-482e-8ac2-22f709c58b4f&type=display. Accessed November 13, 2023.
[26] Evolus Inc. Label: prabotulinumtoxinA-xvfs (JEUVEAU). April 2021. https://dailymed.nlm.nih.gov/dailymed/fda/fdaDrugXsl.cfm?setid=17a914c1-e54b-4b50-965d-b0fd9111bba4&type=display. Accessed November 13, 2023.
[27] Revance Therapeutics, Inc. Label: daxibotulinumtoxinA-lanm (DAXXIFY). August 2023. https://www.accessdata.fda.gov/drugsatfda_docs/label/2023/761127s002lbl.pdf. Accessed November 13, 2023.
[28] Coté TR, Mohan AK, Polder JA, et al. Botulinum toxin type A injections: adverse events reported to the US Food and Drug Administration in therapeutic and cosmetic cases. J Am Acad Dermatol. 2005;53(3):407-415.
[29] Department of Justice. Allergan agrees to plead guilty and pay $600 million to resolve allegations of off-label promotion of Botox. September 1, 2010. https://www.justice.gov/opa/pr/allergan-agrees-plead-guilty-and-pay-600-million-resolve-allegations-label-promotion-botox. Accessed November 13, 2023.
[30] Sifferlin A. Botox: The drug that’s treating everything. Time. January 5, 2017. https://time.com/magazine/south-pacific/4623839/january-16th-2017-vol-189-no-3-asia-europe-middle-east-and-africa-south-pacific/. Accessed November 13, 2023.
[31] Persistence Market Research. Botulinum toxin market. https://www.persistencemarketresearch.com/market-research/botulinum-toxin-market.asp. Accessed November 13, 2023.
[32] American Society of Plastic Surgeons. Plastic surgery statistics report. 2020. http://www.plasticsurgery.org/documents/News/Statistics/2020/plastic-surgery-statistics-full-report-2020.pdf. Accessed November 13, 2023.
[33] Cummins E. Against “preventative” botox. August 15, 2022. https://slate.com/technology/2022/08/preventative-botox-safe-healthy.html. Accessed November 13, 2023.
[34] Rao P. Allergan refocuses Botox strategy on millennials. Glossy. January 28, 2019. https://www.glossy.co/beauty/allergan-refocuses-botox-strategy-on-millennials/. Accessed November 13, 2023.
[35] Ockerman E. Botox company steers ads toward men in face of more competition. May 1, 2018. https://www.thestar.com/business/2018/05/01/botox-company-gears-ads-toward-men-in-face-of-more-competition.html. Accessed November 13, 2023.
[36] Public Citizen. Petition requesting regulatory action concerning the spread of botulinum toxin (Botox, Myobloc) to other parts of the body. January 23, 2008. https://www.citizen.org/article/petition-requesting-regulatory-action-concerning-the-spread-of-botulinum-toxin-botox-myobloc-to-other-parts-of-the-body/. Accessed November 13, 2023.
[37] U.S. Food and Drug Administration. Early communication about an ongoing safety review botox and botox cosmetic (botulinum toxin type A) and myobloc (botulinum toxin type B). Plast Surg Nurs. 2008;28(3):150-151.
[38] Food and Drug Administration. Response to Public Citizen’s 2008 petition requesting regulatory action concerning the spread of botulinum toxin (Botox, Myobloc) to other parts of the body. Docket no. FDA-2008-P-0061. April 30, 2009. https://www.citizen.org/wp-content/uploads/FDA_April-2009.-Response-to-Public-Citizen-petition.pdf. Accessed November 13, 2023.
[39] Allergan, Inc. Label: onabotulinumtoxinA (BOTOX COSMETIC). July 2009. https://www.accessdata.fda.gov/drugsatfda_docs/label/2009/103000s5109s5210lbl.pdf. Accessed November 13, 2023.
[40] Allergan, Inc. Label: onabotulinumtoxinA (BOTOX COSMETIC). February 2021. https://dailymed.nlm.nih.gov/dailymed/fda/fdaDrugXsl.cfm?setid=485d9b71-6881-42c5-a620-a4360c7192ab&type=display. Accessed November 13, 2023.
[41] Wells V. Allergan, Inc. Case number CIV-12-973-C (United States District Court for the Western of Oklahoma). Casetext. January 31, 2013. https://casetext.com/case/wells-v-allergan. Accessed November 13, 2023.
[42] Cronin Fisk M. Allergan settles Oklahoma botox case during trial. February 27, 2013. https://www.drug-injury.com/drug_injury/2013/02/allergan-settles-oklahoma-botox-case-during-trial.html. Accessed November 13, 2023.
[43] European Medicines Agency. Assessment report: botulinum toxin type A (NUCEIVA). July 25, 2019. https://www.ema.europa.eu/en/documents/assessment-report/nuceiva-epar-public-assessment-report_en.pdf. Accessed November 13, 2023.
[44] AbbVie Inc. Summary of product characteristics for healthcare professionals: botulinum toxin type A (BOTOX). June 2023. https://www.medicines.org.uk/emc/product/859/smpc/print. Accessed November 13, 2023.
[45] Rao AK, Lin NH, Jackson KA, et al. Clinical characteristics and ancillary test results among patients with botulism—United States, 2002–2015. Clin Infect Dis. 2017;66(Suppl_1):S4-S10.
[46] Fung HT, Chan KM, Lam SKT. A review on iatrogenic botulism. Hong Kong J Emerg Med. 2020;27(6):356-367.
[47] Rao AK, Sobel J, Chatham-Stephens K, Luquez C. Clinical guidelines for diagnosis and treatment of botulism, 2021. MMWR Recomm Rep. 2021;70(2):1-30.
[48] Fung HT, Chan KM, Lam SKT. A review on iatrogenic botulism. Hong Kong J Emerg Med. 2020;27(6):356-367.
[49] Iancu MS, Spatariu LE, Hurduc V, Plesca DA. Iatrogenic botulism – a possible cause of generalised muscular weakness in child. Ro J Pediatr. 2020;69(3):261-264.
[50] Baig MA, Nogar J. A case of asymmetric cranial nerve palsy due to iatrogenic botulism. Asia Pac J Med Toxicol. 2021;10(4):149-150.
[51] Hovseth K, Badillo R, Schaeffer S, et al. Atypical botulism presentation following botulinum toxin with phenol therapy. In: North American Congress of Clinical Toxicology. Washington, D.C.; 2011:561.
[52] Bai L, Peng X, Liu Y, et al. Clinical analysis of 86 botulism cases caused by cosmetic injection of botulinum toxin (BoNT). Med. 2018;97(34):e10659.
[53] AbbVie Inc. Summary of product characteristics for healthcare professionals: botulinum toxin type A (BOTOX). June 2023. https://www.medicines.org.uk/emc/product/859/smpc/print. Accessed November 13, 2023.
[54] Girlanda P, Vita G, Nicolosi C, et al. Botulinum toxin therapy: distant effects on neuromuscular transmission and autonomic nervous system. J Neurol Neurosurg Psychiatry. 1992;55(9):844-845.
[55] Antonucci F, Rossi C, Gianfranceschi L, et al. Long-distance retrograde effects of botulinum neurotoxin A. J Neurosci. 2008;28(14):3689-3696.
[56] Bomba-Warczak E, Vevea JD, Brittain JM, et al. Interneuronal transfer and distal action of tetanus toxin and botulinum neurotoxins a and D in central neurons. Cell Rep. 2016;6(7):1974-1987.
[57] Rao AK, Sobel J, Chatham-Stephens K, Luquez C. Clinical guidelines for diagnosis and treatment of botulism, 2021. MMWR Recomm Rep. 2021;70(2):1-30.
[58] Halai UA, Terashita D, Kim M, et al. Notes from the field: Intestinal colonization and possible iatrogenic botulism in mouse bioassay-negative serum specimens – Los Angeles county, California, November 2017. MMWR Morb Mortal Wkly Rep. 2018;67(43):1221-1222.
[59] Iancu MS, Spatariu LE, Hurduc V, Plesca DA. Iatrogenic botulism – a possible cause of generalised muscular weakness in child. Ro J Pediatr. 2020;69(3):261-264.
[60] Ghasemi M, Norouzi R, Salari M, Asadi B. Iatrogenic botulism after the therapeutic use of botulinum toxin-A: a case report and review of the literature. Clin Neuropharmacol. 2012;35(5):254-257.
[61] Verity R, Schellenberg K. Botulism-like syndrome following botulinum-A injection for hyperhidrosis and fluoroquinolone use. Can J Neurol Sci. 2022;49(1):154-156.
[62] Iancu MS, Spatariu LE, Hurduc V, Plesca DA. Iatrogenic botulism – a possible cause of generalised muscular weakness in child. Ro J Pediatr. 2020;69(3):261-264.
[63] Roche N, Schnitzler A, Genêt FF, et a. Undesirable distant effects following botulinum toxin type A injection. Clin Neuropharmacol. 2008;31(5):272-280.
[64] Lange DJ, Brin MF, Warner CL, et al. Distant effects of local injection of botulinum toxin. Muscle Nerve. 1987;10(6):552-555.
[65] Centers for Disease Control and Prevention. National botulism surveillance system overview. March 2012. https://www.cdc.gov/ncezid/dfwed/PDFs/bot-overview_508c.pdf. Accessed November 13, 2023.
[66] Young DL, Halstead LA. Pyridostigmine for reversal of severe sequelae from botulinum toxin injection. J Voice. 2014;28(6):830-834.
[67] Sobel J, Rao AK. Making the best of the evidence: Toward national clinical guidelines for botulism. Clin Infect Dis. 2017;66(Suppl 1):S1-S3.
[68] Cherington M. Botulism: update and review. Semin Neurol. 2004;24(2):155-163.
[69] Rao AK, Sobel J, Chatham-Stephens K, Luquez C. Clinical guidelines for diagnosis and treatment of botulism, 2021. MMWR Recomm Rep. 2021;70(2):1-30.
[70] Cangene Corporation. Label: Botulism antitoxin heptavalent (BAT). March 2017. https://www.fda.gov/media/85514/download. Accessed November 13, 2023.
[71] Rao AK, Sobel J, Chatham-Stephens K, Luquez C. Clinical guidelines for diagnosis and treatment of botulism, 2021. MMWR Recomm Rep. 2021;70(2):1-30.
[72] Cangene Corporation. Label: Botulism antitoxin heptavalent (BAT). March 2017. https://www.fda.gov/media/85514/download. Accessed November 13, 2023.
[73] Fan KL, Wang YL, Chu G, Leung LP. Delayed antitoxin treatment of two adult patients with botulism after cosmetic injection of botulinum type A toxin. J Emerg Med. 2016;51(6):677-679.
[74] Cangene Corporation. Label: Botulism antitoxin heptavalent (BAT). March 2017. https://www.fda.gov/media/85514/download. Accessed November 13, 2023.
[75] Rao AK, Sobel J, Chatham-Stephens K, Luquez C. Clinical guidelines for diagnosis and treatment of botulism, 2021. MMWR Recomm Rep. 2021;70(2):1-30.
[76] Halai UA, Terashita D, Kim M, et al. Notes from the field: Intestinal colonization and possible iatrogenic botulism in mouse bioassay-negative serum specimens – Los Angeles county, California, November 2017. MMWR Morb Mortal Wkly Rep. 2018;67(43):1221-1222.
[77] Fan KL, Wang YL, Chu G, Leung LP. Delayed antitoxin treatment of two adult patients with botulism after cosmetic injection of botulinum type A toxin. J Emerg Med. 2016;51(6):677-679.
[78] Mohammed EA, Salah Eldin HM. Highlights of serious distant effects after local injection of botulinum toxin therapy: A report of pediatric case. Int J Case Rep Images. 2020;11(June 2):101127Z01EM2020.
[79] Oner OG, Gudek HC, Cetin OE, Demir S. Iatrogenic botulism: A case treated with botulinum antitoxin. Clin Neuropharmacol. 2023;46(2):82-84.
[80] Fan KL, Wang YL, Chu G, Leung LP. Delayed antitoxin treatment of two adult patients with botulism after cosmetic injection of botulinum type A toxin. J Emerg Med. 2016;51(6):677-679.
[81] Centers for Disease Control and Prevention. Botulism in the United States, 1899-1996. Handbook for Epidemiologists, Clinicians, and Laboratory Workers. 1998. https://www.cdc.gov/botulism/pdf/bot-manual.pdf. Accessed November 13, 2023.
[82] Halai UA, Terashita D, Kim M, et al. Notes from the field: Intestinal colonization and possible iatrogenic botulism in mouse bioassay-negative serum specimens – Los Angeles county, California, November 2017. MMWR Morb Mortal Wkly Rep. 2018;67(43):1221-1222.
[83] Baig MA, Nogar J. A case of asymmetric cranial nerve palsy due to iatrogenic botulism. Asia Pac J Med Toxicol. 2021;10(4):149-150.
[84] Rao AK, Sobel J, Chatham-Stephens K, Luquez C. Clinical guidelines for diagnosis and treatment of botulism, 2021. MMWR Recomm Rep. 2021;70(2):1-30.
[85] Gostimir M, Liou V, Yoon MK. Safety of botulinum toxin A injections for facial rejuvenation: A meta-analysis of 9,669 patients. Ophthalmic Plast Reconstr Surg. 2023;39(1):13-25.
[86] Duarte GS, Rodrigues FB, Marques RE, et al. Botulinum toxin type A therapy for blepharospasm. Cochrane Database Syst Rev. 2020;11(11):CD004900.
[87] Rodrigues FB, Duarte GS, Marques RE, et al. Botulinum toxin type A therapy for cervical dystonia. Cochrane Database Syst Rev. 2020;2020(11):CD003633.
[88] Yiannakopoulou E. Serious and long-term adverse events associated with the therapeutic and cosmetic use of botulinum toxin. Pharmacology. 2015;95(1-2):65-69.
[89] Tugnoli V, Eleopra R, Quatrale R, et al. Botulism-like syndrome after botulinum toxin type A injections for focal hyperhidrosis. Br J Dermatol. 2002;147(4):808-809.
[90] Goldstein EM. Safety of high-dose botulinum toxin type A therapy for the treatment of pediatric spasticity. J Child Neurol. 2006;21(3):189-192.
[91] Crowner BE, Brunstrom JE, Racette BA. Iatrogenic botulism due to therapeutic botulinum toxin a injection in a pediatric patient. Clin Neuropharmacol. 2007;30(5):310-313.
[92] Howell K, Selber P, Graham HK, et al. Botulinum neurotoxin A: an unusual systemic effect. J Paediatr Child Heal. 2007;43(6):499-501.
[93] Partikian A, Mitchell WG. Iatrogenic botulism in a child with spastic quadriparesis. J Child Neurol. 2007;22(10):1235-1237.
[94] Tirado-Sanchez A, Ponce-Olivera R. Botulism-like syndrome without cholinergic involvement: A case report and two recommendations to avoid it. Indian J Dermatol. 2007;52(3):163-164.
[95] Partikian A, Mitchell WG. Iatrogenic botulism in a child with spastic quadriparesis. J Child Neurol. 2007;22(10):1235-1237.
[96] Mezaki T. Unexpected serious adverse reactions to the therapy with botulinum toxin might be due to hypersensitivity. Med Hypotheses. 2009;73(6):1076-1077.
[97] Crowner BE, Torres-Russotto D, Carter AR, et al. Systemic weakness after therapeutic injections of botulinum toxin a: a case series and review of the literature. Clin Neuropharmacol. 2010;33(5):243-247.
[98] Petrucci A, Lispi L. Generalized botulism after botulinum toxin-A injection. Mov Disord. 2011;26(Suppl. 2):S40-S41.
[99] Baig MA, Nogar J. A case of asymmetric cranial nerve palsy due to iatrogenic botulism. Asia Pac J Med Toxicol. 2021;10(4):149-150.
[100] Haddad R, Rech C, Durand MC, et al. Botulism-like syndrome or widespread diffusion syndrome after injection of botulinum toxin A for neurogenic detrusor overactivity. Ann Phys Rehabil Med. 2012;55(Supplement 1):e340.
[101] Para K, Zivari K, Santoshi R, et al. Iatrogenic botulism. Chest. 2020;158(4):A930.
[102] Willis AW, Crowner B, Brunstrom JE, et al. High dose botulinum toxin A for the treatment of lower extremity hypertonicity in children with cerebral palsy. Dev Med Child Neurol. 2007;49(11):818-822.
[103] Verity R, Schellenberg K. Botulism-like syndrome following botulinum-A injection for hyperhidrosis and fluoroquinolone use. Can J Neurol Sci. 2022;49(1):154-156.
[104] Bakheit AM, Ward CD, McLellan DL. Generalised botulism-like syndrome after intramuscular injections of botulinum toxin type A: a report of two cases. J Neurol Neurosurg Psychiatry. 1997;62(2):198.
[105] Bhatia KP, Münchau A, Thompson PD, et al. Generalised muscular weakness after botulinum toxin injections for dystonia: a report of three cases. J Neurol Neurosurg Psychiatry. 1999;67(1):90-93.
[106] Cobb DB, Watson WA, Fernandez MC. Botulism-like syndrome after injections of botulinum toxin. Vet Hum Toxicol. 2000;42(3):163.
[107] Mezaki T. Unexpected serious adverse reactions to the therapy with botulinum toxin might be due to hypersensitivity. Med Hypotheses. 2009;73(6):1076-1077.
[108] Coban A, Matur Z, Hanagasi HA, Parman Y. Iatrogenic botulism after botulinum toxin type A injections. Clin Neuropharmacol. 2010;33(3):158-160.
[109] Ghasemi M, Norouzi R, Salari M, Asadi B. Iatrogenic botulism after the therapeutic use of botulinum toxin-A: A case report and review of the literature. Clin Neuropharmacol. 2012;35(5):254-257.
[110] Perot A, Panguy B, Durufle A, et al. Botulism like syndrome about one case. Ann Phys Rehabil Med. 2015;58(Supplement 1):e83-e84.
[111] Halai UA, Terashita D, Kim M, et al. Notes from the field: Intestinal colonization and possible iatrogenic botulism in mouse bioassay-negative serum specimens – Los Angeles county, California, November 2017. MMWR Morb Mortal Wkly Rep. 2018;67(43):1221-1222.
[112] Leonardi L, Haggiag S, Petrucci A, Lispi L. Electrophysiological abnormalities in iatrogenic botulism: Two case reports and review of the literature. J Clin Neurosci. 2019;60(Feb):138-141.
[113] Iancu MS, Spatariu LE, Hurduc V, Plesca DA. Iatrogenic botulism – a possible cause of generalised muscular weakness in child. Ro J Pediatr. 2020;69(3):261-264.
[114] McGuinn KP, Santa E, Saedi N. Distant onabotulinumtoxinA spread after cosmetic dosing to facial rhytides. Skinmed. 2019;17(2):137-138.
[115] Kouris A, Agiasofitou E, Gregoriou S, et al. Generalized neurological symptoms following treatment of focal hyperhidrosis with botulinum toxin A. Int J Dermatol. 2014;53(11):e544-e547.
[116] Obagi S, Golubets K. Mild to moderate dysphagia following very low-dose abobotulinumtoxin a for platysmal bands. J Drugs Dermatol. 2017;16(9):929-930.
[117] Turone F. Botulism cases linked to intragastric injections for obesity. Medscape. March 24, 2023. https://www.medscape.com/viewarticle/990094. Accessed November 13, 2023.
[118] Bensen GP, Rutherford CC, Gardner TB. Systemic botulism toxicity caused by pyloric botox injection to treat gastroparesis. Case Rep Gastroenterol. 2020;14(2):373-376.
[119] Coté TR, Mohan AK, Polder JA, et al. Botulinum toxin type A injections: Adverse events reported to the US Food and Drug Administration in therapeutic and cosmetic cases. J Am Acad Dermatol. 2005;53(3):407-415.
[120] Hristova AH, Joseph LN, Sathe SA, et al. Severe nervous system complications after botulinum type A therapy: Three case reports with reviews of FDA-reported nervous system adverse effects. PM R. 2012;4(8):613-623.
[121] Phadke CP, Balasubramanian CK, Holz A, et al. Adverse clinical effects of botulinum toxin intramuscular injections for spasticity. Can J Neurol Sci. 2015;43(2):298-310.
[122] Montastruc J, Marque P, Moulis F, et al. Adverse drug reactions of botulinum neurotoxin type A in children with cerebral palsy: a pharmaco-epidemiological study in VigiBase. Dev Med Child Neurol. 2017;59(3):329-334.
[123] Yiannakopoulou E. Botulinum toxin and safety issues in cerebral palsy. Dev Med Child Neurol. 2017;59(3):245.
[124] Ahsanuddin S, Roy S, Nasser W, et al. Adverse events associated with botox as reported in a Food and Drug Administration Database. Aesthetic Plast Surg. 2021;45(3):1201-1209.
[125] Zargaran D, Zoller FE, Zargaran A, Mosahebi A. Complications of facial cosmetic botulinum toxin A injection: analysis of the UK Medicines & Healthcare Products Regulatory Agency registry and literature review. J Plast Reconstr Aesthet Surg. 2022;75(1):392-401.
[126] Food and Drug Administration. Response to Public Citizen’s 2008 petition requesting regulatory action concerning the spread of botulinum toxin (Botox, Myobloc) to other parts of the body. Docket no. FDA-2008-P-0061. April 30, 2009. https://www.citizen.org/wp-content/uploads/FDA_April-2009.-Response-to-Public-Citizen-petition.pdf. Accessed November 13, 2023.
[127] Rao AK, Lin NH, Jackson KA, et al. Clinical characteristics and ancillary test results among patients with botulism—United States, 2002–2015. Clin Infect Dis. 2017;66(Suppl_1):S4-S10.
[128] Phadke CP, Balasubramanian CK, Holz A, et al. Adverse clinical effects of botulinum toxin intramuscular injections for spasticity. Can J Neurol Sci. 2015;43(2):298-310.
[129] Note, these terms use British English in FAERS per MedDRA coding.
[130] Hazell L, Shakir SAW. Under-reporting of adverse drug reactions: A systematic review. Drug Saf. 2006;29(5):385-396.
[131] Aimer O, Roche N, Michelon H, et al. Low prevalence of botulinum toxin-A adverse effects: Good safety profile or underreporting? J Nov Physiother Phys Rehabil. 2017;4(4):090-093.
[132] Centers for Disease Control and Prevention. National botulism surveillance system overview. March 2012. https://www.cdc.gov/ncezid/dfwed/PDFs/bot-overview_508c.pdf. Accessed November 13, 2023.
[133] Halai UA, Terashita D, Kim M, et al. Notes from the field: Intestinal colonization and possible iatrogenic botulism in mouse bioassay-negative serum specimens – Los Angeles county, California, November 2017. MMWR Morb Mortal Wkly Rep. 2018;67(43):1221-1222.
[134] Food and Drug Administration. Guidance for industry: Warnings and precautions, contraindications, and boxed warning sections of labeling for human prescription drug and biological products — content and format. October 2011. https://www.fda.gov/media/71866/download. Accessed November 13, 2023.
[135] Food and Drug Administration. Response to Public Citizen’s 2008 petition requesting regulatory action concerning the spread of botulinum toxin (Botox, Myobloc) to other parts of the body. Docket no. FDA-2008-P-0061. April 30, 2009. https://www.citizen.org/wp-content/uploads/FDA_April-2009.-Response-to-Public-Citizen-petition.pdf. Accessed November 13, 2023.
[136] AbbVie Corporation. Product monograph: onabotulinumtoxinA (BOTOX COSMETIC). November 4, 2022. https://www.abbvie.ca/content/dam/abbvie-dotcom/ca/en/documents/products/BOTOXCOSMETIC_PM_EN.pdf. Accessed November 13, 2023.
[137] Allergan Inc. Monograph: Product monograph: onabotulinumtoxinA (BOTOX). March 11, 2021. https://pdf.hres.ca/dpd_pm/00060199.PDF. Accessed November 13, 2023.
[138] AbbVie Inc. Summary of product characteristics for healthcare professionals: botulinum toxin type A (BOTOX). June 2023. https://www.medicines.org.uk/emc/product/859/smpc/print. Accessed November 13, 2023.
[139] Public Citizen. Petition requesting regulatory action concerning the spread of botulinum toxin (Botox, Myobloc) to other parts of the body. January 23, 2008. https://www.citizen.org/article/petition-requesting-regulatory-action-concerning-the-spread-of-botulinum-toxin-botox-myobloc-to-other-parts-of-the-body/. Accessed November 13, 2023.
[140] Food and Drug Administration. Response to Public Citizen’s 2008 petition requesting regulatory action concerning the spread of botulinum toxin (Botox, Myobloc) to other parts of the body. Docket no. FDA-2008-P-0061. April 30, 2009. https://www.citizen.org/wp-content/uploads/FDA_April-2009.-Response-to-Public-Citizen-petition.pdf. Accessed November 13, 2023.