BioNTech and Pfizer’s BNT162 Vaccine Patent Landscape
By Mario Gaviria and Burcu Kilic
Safe and effective vaccines are key to combating the Covid-19 pandemic; however, patents and other intellectual property claims directed at vaccine technologies create legal barriers for equitable access and fair allocation. No corporation produces at scale to supply the world. Providing timely global access will depend in significant part on increasing supply, including by transferring technology to qualified manufacturers. Much of this technology is claimed as patented, proprietary, or confidential in nature.
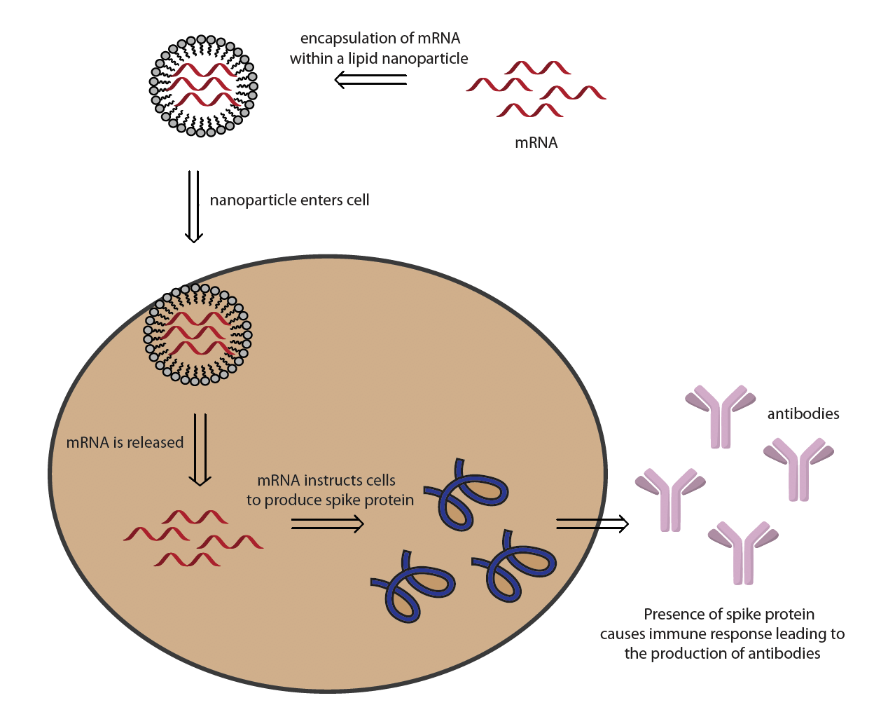
German company BioNTech and its U.S. partner Pfizer’s2 vaccine candidate, BNT162 SARS-CoV-2, employs the use of lipid nanoparticle (NP) technology to deliver mRNA to cells. Once the lipid nanoparticle is injected into a patient, it travels into the cells and instructs them to produce the SARS-CoV-2 spike protein. The presence of this coronavirus protein is thought to trigger an immune response leading to the production of antibodies.3 If the patient is infected with coronavirus, the antibodies will identify and bind to the virus, which triggers a series of events resulting in the elimination of the virus.
BNT162 is in Phase 3 clinical trials. Pfizer announced promising but preliminary trial results on November 9th.4
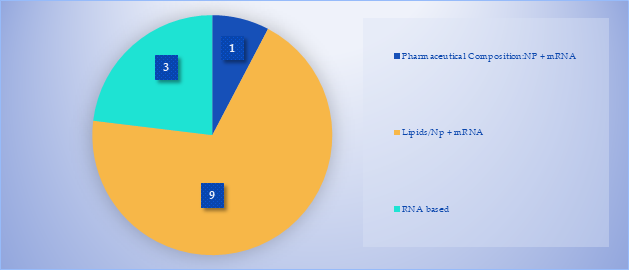
We identified several patents claimed by BioNTech relating to the pertinent vaccine technologies.5 We placed them in three groups based on their description and their primary independent claim:
- Patents directed at RNA
- Patents directed at Lipids/NP + mRNA
- Patents specifically directed at pharmaceutical compositions involving lipid NP + mR
Below is our non-exhaustive list. In a recent financial statement, BioNTech suggested that its patent claims extend to mRNA structure, formulations, and manufacturing, and relies on trade secrets and confidential know-how to protect aspects of mRNA manufacturing technologies.6
| Patent/Published Application | Applicant/Assignee | Filing Date | Status | Invention Type |
| US 10,576,146 | BioNTech | March 15, 2018 | Active | Lipids/NP + mRNA |
| US 10,485,884 | BioNTech | March 5, 2013 | Active | Lipids/NP + mRNA |
| US 9,950,065 | BioNTech | September 26, 2013 | Active | Lipids/NP + mRNA |
| US2020/0155671 | BioNTech | January 22, 2020 | Pending | Lipids/NP + mRNA |
| US2020/0197508 | BioNTech | March 21, 2018 | Pending | RNA immune response |
| US2019/0153428 | BioNTech | August 24, 2016 | Pending | RNA immunogenicity |
| US2019/0321458 | BioNTech | July 14, 2017 | Pending | PC: Lipids/NP + mRNA |
| US2018/0263907 | BioNTech | March 30,2016 | Pending | Lipids/NP + mRNA |
| US2017/0273907 | BioNTech | September 17, 2015 | Pending | Lipids/NP + mRNA |
| US2014/0030808 | BioNTech | December 2, 2011 | Pending | RNA expression |
| WO2016/156398 | BioNTech | March 30,2016 | Published | Lipids/NP + mRNA |
| WO2015/043613 | BioNTech | September 26, 2013 | Published | Lipids/NP + mRNA |
| WO2013/087083 | BioNTech | December 15, 2011 | Published | Lipids/NP + mRNA |
2 All patents and patent applications identified in this study were claimed by BioNTech indicating that they are the inventor of the relevant vaccine technology, while Pfizer is acting as the innovator and leading the large-scale manufacturing, development, and regulatory approval process.
3 https://www.nejm.org/doi/10.1056/NEJMoa2027906
4 https://www.pfizer.com/news/press-release/press-release-detail/pfizer-and-biontech-announce-vaccine-candidate-against
5 Pharmaceutical companies are not the only claimants of key technology. The U.S. government claims a patent on a key technology which may be relevant for BioNTech and Pfizer to stabilize the spike protein. See Public Citizen, Leading COVID-19 Vaccine Candidates Depend on NIH Technology (Nov. 10, 2020), https://www.citizen.org/article/leading-covid-19-vaccines-depend-on-nih-technology/.
6 “Certain of our technologies, including in particular certain proprietary manufacturing processes or technologies and/or neoantigen prediction technologies, are protected as trade secrets”,.BioNTech SE, SEC Filing (July 21 2020), https://www.sec.gov/Archives/edgar/data/1776985/000119312520195911/d939702df1.htm.