CRISPR Gene Editing: the Immediate Future of Bioengineering and Medicine
Health Letter, April 2023
By Michael T. Abrams, M.P.H., Ph.D.
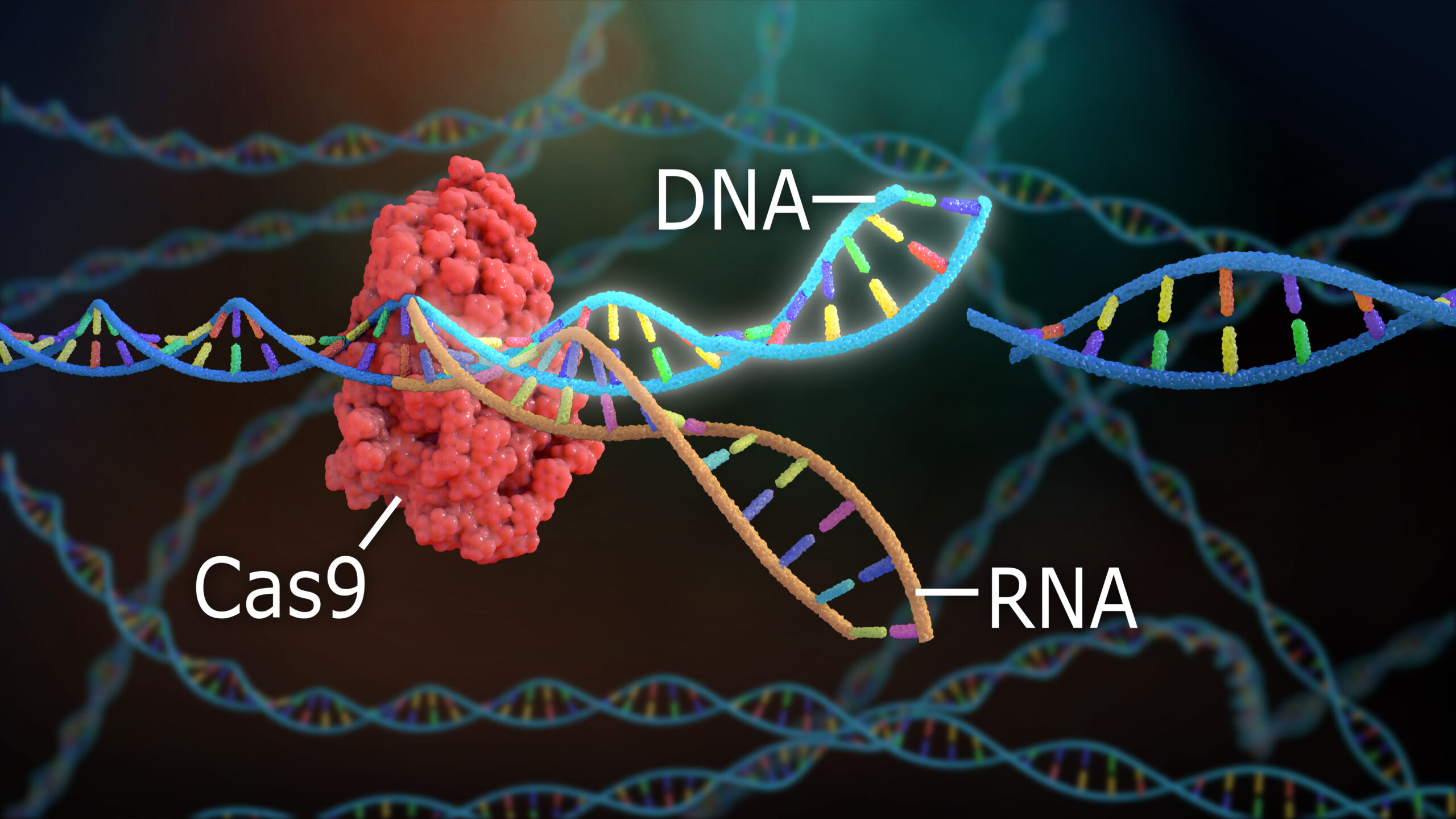
In 2011-2012, two women, Emmanuelle Charpentier, Ph.D., and Jennifer Duodna, Ph.D., directed a small, international team of scientists in the detailing of a naturally occurring enzymatic system that bacteria use to isolate and cut apart the genetic code (DNA) of invading pathogens (for example, viruses).[1] Understanding the workings of a key component of this primordial immune system, referred to as “CRISPR/Cas9” (hereinafter, CRISPR [pronounced “crisper”]) was a tremendous advancement in basic biochemistry and molecular biology, and one that delivered to the world a tool for gene editing in the cells of most living organisms. For first describing the CRISPR, Drs. Charpentier and Duodna were awarded the 2020 Nobel Prize in Chemistry.
In a press release announcing this prestigious award, the Swedish Academy of Sciences wrote:
This [CRISPR] technology has had a revolutionary impact on the life sciences, is contributing to new cancer therapies and may make the dream of curing inherited disease come true.[2]
Since 2012 there has been much activity by scientists, lawyers and investors to develop gene-editing technologies based on CRISPR and to secure intellectual property rights pertaining to those developments. One epicenter of that scientific and financial quest is the Broad Institute in Cambridge, Mass., where Feng Zhang, Ph.D., a researcher who was among the first to deploy CRISPR in human cells, reportedly just established his seventh biotech deal spawned by the CRISPR revolution, bringing the current valuation of his ventures in that domain to over $4.7 billion.[3] Such ventures continue robustly even as intellectual property disputes remain unresolved.[4] One biotech expert actually told the online media outlet STAT, which covers health and medicine, that if a firm hopes to contend in the burgeoning CRISPR space, they need to act “fast, break things, worry about the patent licenses later.”
Certainly, the ongoing evolution of CRISPR has transformed bioscience while sometimes breaking things, including the norms governing human genetic engineering practices. In 2018, a scientist in China, He Jiankui, Ph.D., startled the world by announcing that he had CRISPR-altered embryos that ultimately became live-born human twins.[5] Those twins, whose identity and full health status remain unknown to most of the world,[6],[7],[8] were said by Dr. He to have been given genetic rearrangements that protect them from HIV/AIDS infection. Many in the scientific world responded critically, some with indignation.[9] In 2019, the Chinese government jailed He for three years and fined him $445,000 for “practicing medicine without a license.” In late February 2023, he announced that he had obtained a Hong Kong visa and planned to continue his career while respecting “ethics codes and international consensus on scientific research,” but his visa was abruptly revoked by Chinese authorities with little explanation.
So, what is CRISPR, and why is the world so intrigued by it? Below is a brief description of this gene-editing system, followed by notations regarding the human medical potential and status of this revolutionary technology.
What is CRISPR?
CRISPR is an acronym that stands for Clustered Regularly Interspaced Short Palindromic Repeats because early evidence of this system came from scientists who observed that bacteria contain DNA sequences with a repeating pattern containing bits of coding DNA that correspond to a genetic library of external viruses that threaten the bacteria as well as enzymes the bacteria routinely use (with the library of external viruses as guides) to neutralize those viral threats.
Charpentier, Duodna and colleagues first detailed the full structure of CRISPR-associated protein complex number 9 (Cas9) that eventually won them the Nobel Prize. They further demonstrated that they could alter and affix tailor-made, single-stranded genetic code (RNA) fragments to the Cas9 so as to initiate a precise cut in bacterial DNA. This naturally led Charpentier, Duodna and many others to realize that their discovery would open up new opportunities for gene editing, because where a precise cut can be made, precise repairs, including the insertion of a new or corrected gene, also can occur.
What is the potential of CRISPR technologies to combat human disease?
In 2013, researchers led by Feng Zhang adapted the CRISPR technology to edit genes in human cells.[10] This required some incremental innovations because human cells are more complex than bacterial cells. Equally important, compared with earlier commonly used gene-editing techniques, CRISPR affords “…easy handling, higher efficiency, limited off-target sites, lower cost, and the ability of altering different genomic sites at the same time…”[11]
Accordingly, CRISPR-based research has accelerated the pace at which scientists can silence (“knockout”), input (add, “knock in”) or modulate (control the amount of) gene expression in most living cells.[12],[13] This sort of “on/off” or “dimmer-switch” control of individual gene translation is not only useful to directly address faulty gene expression, it also is a way to precisely engineer animal models for such genetic diseases.
Vis-à-vis these CRISPR-facilitated genetic switches, there are now numerous studies in progress in which CRISPR is being used to better understand and eventually treat a variety of genetic-based illnesses, including various cancers; certain congenital or otherwise chronic neurologic diseases such as Alzheimer’s disease, Huntington’s disease and fragile X syndrome; and a variety of other human inherited diseases such as cystic fibrosis and Duchenne muscular dystrophy.[14],[15]
One 2023 review tabulated 20 diseases that were the subject of clinical trials involving CRISPR-based interventions.[16] Sixteen were for various cancers (for example, leukemia, cervical, prostate and lung cancer), and other diseases included were beta-thalassemia (a genetic disorder that results in insufficient hemoglobin production), keratitis (inflamed cornea) related to herpes infection, Leber congenital amaurosis (retinal disease) and hereditary angioedema (severe, recurrent swelling, typically of the limbs, face, intestinal track and airway) Except for thalassemia, those trials all were in early phase I or II. Another hemoglobin disorder, sickle cell disease, also is being targeted with CRISPR by removing blood precursor cells from the patient and gene-editing them ex vivo (outside the body), then returning them back into the body.[17] A specific “breakthrough” cancer therapy application of CRISPR involves a technique referred to as “chimeric antigen receptor-T cell” (CAR-T) therapy, which involves genetically modifying immune cells to selectively fight cancer cells.[18]
Despite the promise and comparative precision of CRISPR, errors can occur, including what are called “off-target” rearrangements, a euphemism for unintended genetic changes. In some cases, those changes may be benign, but in others they may be harmful. Accordingly, regulatory oversight, especially by the U.S. Food and Drug Administration (FDA), must be vigilant when CRISPR-based technologies are considered for widespread use.
The FDA’s March 2022 draft guidance regarding gene editing indicates that although the potential benefits of products resulting from this rapidly evolving technology may be straight-forward, associated risks are poorly understood, including the risks of off-target editing and other unintended consequences that may manifest in the near- or long-term.[19] The FDA guidance further notes that in vivo (within the patient’s body) gene editing is on the horizon, facilitated by nano delivery methods that were critical to the success of the mRNA COVID-19 vaccines.
Ethical concerns, “designer babies”
Germline cell (egg or sperm) editing is also greatly accelerated by CRISPR, including the production of embryos that can be implanted using long-established in vitro fertilization (IVF) procedures leading to live births.
At present, human CRISPR or any human germline gene-editing practices are mostly limited to laboratory experiments where early, nonviable embryos are studied and not implanted to create a pregnancy.
Still, many believe that in certain circumstances, CRISPR-engineered human babies are desirable to ease suffering (for example, by decreasing the risk of devastating diseases like Huntington’s disease or cystic fibrosis) or, more controversially, to advance the general fitness (for example, strength, intelligence) of offspring.
Notably, as of 2021, the World Health Organization does not call for a prohibition on germline editing but instead encourages, and itself is facilitating, the development of guidelines to conduct such experiments safely and equitably.[20] Separately, a 2018 webpage posted by the National Human Genome Research Institute of the U.S. National Institutes of Health was similarly cautious but otherwise apparently open to germline editing, including that which may lead to genetically altered babies:
Bioethicists and researchers generally believe that human genome editing for reproductive purposes should not be attempted at this time, but that studies that would make gene therapy safe and effective should continue.[21]
The future
CRISPR technology, first detailed to the world in just 2012, has advanced human genetic engineering tremendously. It has become an invaluable and rapidly evolving tool that is akin to a precise and programmable genetic scalpel that can correct or introduce desired DNA code into most living organisms. It is being deployed to create drugs or modified auto-transplant cells, and it is also a certain pathway towards “designer babies,” well beyond the questionable endeavors of one scientist in China. To ignore CRISPR is to ignore the current emergence of much basic and practical bioscientific know-how, including the emergence of potentially important, and sometimes very risky, therapeutic and preventative interventions.
For a rich historical narrative surrounding CRISPR, consider reading Walter Isaacson’s biography about Nobel Laureate Jennifer Duodna.[22] Isaacson’s 500-page book is written for nonscientists, and it nicely explains and contextualizes Duodna’s technical and personal journey from her childhood/adolescence in Hawaii (when and where she first read about Watson and Crick’s discovery of the DNA double helix) to a distinguished professorship (including Nobel and responding to COVID-19) at University of California, Berkeley.
For a somewhat sensationalized, movie-form description of He Jiankui’s controversial CRISPR-baby experiment, consider watching the 2022 docudrama, “Make People Better” (directed by Cody Sheehy). Despite its Hollywood-style brazenness, this film is evocative and just 1 hour and 23 minutes in length.
References
[1] Jinek M, Chylinski K, Fonfara I, et al. A programmable dual-RNA-guided DNA endonuclease in adaptive bacterial immunity. Science. 2012;337(6096):816-21.
[2] Press Release: The Nobel Prize in Chemistry 2020. The Royal Swedish Academy of Sciences, October 7, 2020.
[3] DeAngelis A. CRISPR pioneer Feng Zhang launches new genetic delivery starteup with $193 million. STAT. February 16, 2023.
[4] Mast J, DeAngelis A and Molteni M. CRISPR patent fight redux? A new battle is brewing among biotechs over next-gen gene-editing tools. STAT. March 1, 2023.
[5] Kirksey E. Guest essay: Does gene editing have a future in reproductive medicine? New York Times. March 4, 2023.
[6] Molteni M. Ahead of genome summit in London, questions linger about CRISPR baby scandal. STAT+. March 3, 2023.
[7] Cohen J. As creator of ‘CRISPR babies’ nears release from prison, where does embryo editing stand? Science. March 21, 2022.
[8] Gutierrez N. What’s next for the gene-edited children from CRISPR trial in China? New Scientist. June 29, 2022.
[9] Leung K, Fujiyama EW. Hong Kong pulls visa for ‘CRISPR babies’ scientist He Jiankui. Associated Press. February 21, 2021.
[10] Cong L, Ran FA, Cox D, et al. Multiplex genome engineering using CRISPR/Cas systems. Science. 2013 Feb 15;339(6121):819-23.
[11] Boti MA, Athanasopoulou K, Adamopoulos PG, et al. Recent advances in genome-engineering strategies. Genes (Basel). 2023;14(1):129. https://doi.org/10.3390/genes14010129.
[12] Ibid.
[13] Chavez M, Chen X, Finn PB, Qi LS. Advances in CRISPR therapeutics. Nat Rev Nephrol. 2023 Jan;19(1):9-22.
[14] Boti MA, Athanasopoulou K, Adamopoulos PG, et al. Recent advances in genome-engineering strategies. Genes (Basel). 2023;14(1):129. https://doi.org/10.3390/genes14010129.
[15] Chavez M, Chen X, Finn PB, Qi LS. Advances in CRISPR therapeutics. Nat Rev Nephrol. 2023 Jan;19(1):9-22.
[16] Boti MA, Athanasopoulou K, Adamopoulos PG et al. Recent advances in genome-engineering strategies. Genes (Basel). 2023;14(1):129; https://doi.org/10.3390/genes14010129.
[17] Kolata G. Sickle cell brings mix of anxiety and hope. New York Times. January 17, 2023.
[18] Boti MA, Athanasopoulou K, Adamopoulos PG et al. Recent advances in genome-engineering strategies. Genes (Basel). 2023;14(1):129; https://doi.org/10.3390/genes14010129.
[19] U.S. Department of Health and Human Services. Food and Drug Administration. Center for Biologics Evaluation and Research. “Draft Guidance for Industry: Human Gene Therapy Products Incorporating Human Genome Editing.” March 2022
[20] WHO Expert Advisory Committee on Developing Global Standards for Governance and Oversight of Human Genome Editing. Human Genome Editing: recommendations. Geneva: World Health Organization; 2021. Licence: CC BY-NC-SA 3.0 IGO.
[21] National Institutes of Health. National Human Genome Research Institute. “What are the Ethical Concerns of Genome Editing? August 3, 2017. https://www.genome.gov/about-genomics/policy-issues/Genome-Editing/ethical-concerns. Accessed March 6, 2022.
[22] Isaacson W. The Code Breaker. Simon and Shuster. New York, New York. March 2021.