
A Piece of the COVID Vaccine Recipe
Public Citizen has identified a piece of the Pfizer-BioNTech vaccine recipe. Based on a publicly available contract, Public Citizen has found manufacturing specifications from November 2020 for the vaccine Comirnaty (BNT162b2).[1] This information can help mRNA vaccine scientists around the world by illustrating the kinds of requirements they need to meet critical quality standards. It can also advance mRNA science and, together with the rest of the recipe, help bolster global vaccine production.
Critical Manufacturing Information is Available in a Public Contract
In November 2020, the European Commission signed an agreement with Pfizer and BioNTech to purchase 100 million doses (“the EC Contract”). In April 2021, the Italian public broadcaster RAI published the EC Contract.[2] The RAI story and EC contract were shared widely on social media, including by members of the European parliament and international journalists.[3] In September 2021, Public Citizen reviewed the contract and prepared this analysis documenting the manufacturing specifications contained in the public agreement.[4]
Specifications
A specification is defined as “a list of tests, references to analytical procedures, and appropriate acceptance criteria.” Specifications are “chosen to confirm the quality of the drug substance and drug product.”[5] They focus on the molecular and biological characteristics that have been found useful for assessing product safety and efficacy. Specifications are product-specific, and manufacturers can use different approaches to demonstrate product quality. However, the approach used by one manufacturer can nonetheless be instructive for others: scientists can learn about, quantify, and aim towards a quality target when producing mRNA vaccines.
BNT162b2 Specifications as of November 20, 2020
The EC Contract contains composition and strength, identity, and purity requirements. It names the quality attribute (the characteristic), the analytical procedure (the test), and the acceptance criteria (the test requirements). To our knowledge, the acceptance criteria—or the requirements a batch of vaccine must meet before it can be released—have not been fully described anywhere, except in the EC Contract.
For example, the U.S. Food and Drug Administration (FDA) has only released heavily redacted specifications.[6] The European Medicines Agency (EMA) has released information about quality attributes and analytical procedures, but not acceptance criteria.[7]
Figure 1: Heavily Redacted BNT162b2 Specifications Contained in the FDA Summary Basis for Regulatory Action (Aug 23, 2021)[8]
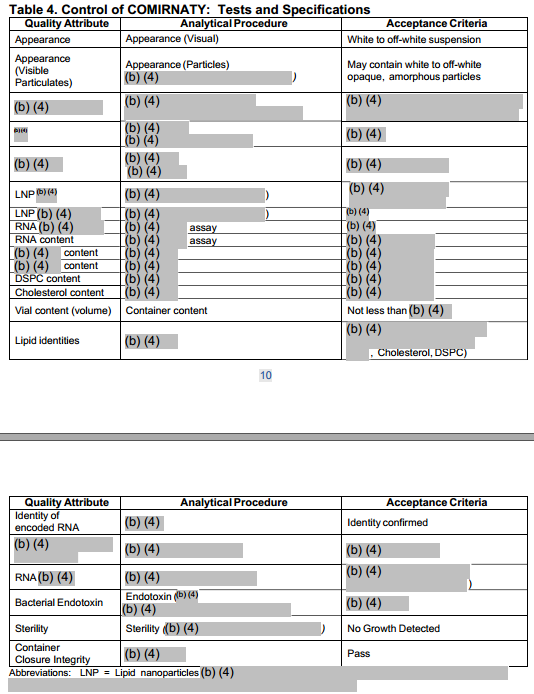
We summarize key specifications contained in the EC Contract below.[9] This information was likely slightly updated since the contract was signed in November, including the introduction of one new specification that was not described in the contract.[10] We note that the information contained in Table 1 below also appears to closely track the redacted information recently released by the FDA (Figure 1). For example, the FDA document lists 21 quality attributes and the EC Contract lists 20 quality attributes.[11]
Table 1: BNT162b2 Drug Product Specifications Contained in the EC Contract (Nov 20, 2020)
| Drug Product Specification | |||
| Quality Attribute | Analytical Procedure | Acceptance Criteria | |
| Composition and Strength | 1. Container content for injections[12] | Volume of injections in containers | “Not less than stated dose” |
| 2. Cholesterol content | High-performance liquid chromatography with charged aerosol detector | Document result: milligram per milliliter | |
| 3. 1,2-distearoyl-snglycero-3-phosphocholine (DSPC) content |
High-performance liquid chromatography with charged aerosol detector | Document result: milligram per milliliter | |
| 4. 2-[(polyethylene glycol)-2000]-N,N-ditetradecylacetamide (ALC-0159) content |
High-performance liquid chromatography with charged aerosol detector | Document result: milligram per milliliter | |
| 5. 4-hydroxybutyl)azanediyl)bis (hexane-6,1- diyl)bis(2-hexyldecanoate) (ALC-3015) content |
High-performance liquid chromatography with charged aerosol detector | Document result: milligram per milliliter | |
| 6. RNA content | Fluorescence assay | ≥ 80% | |
| 7. RNA encapsulation | Fluorescence assay | 0.50 ± 0.13 milligram per milliliter | |
| 8. Lipid nanoparticle polydispersity | Dynamic light scattering | ≤ 0.3 | |
| 9. Lipid nanoparticle size | Dynamic light scattering | ≤ 200 nanometers | |
| 10. Osmolality | Osmometry | 525 ± 100 milliosmoles per kilogram | |
| 11. pH | Potentiometry | 7.4 ± 0.5 | |
| 12. Subvisible particles | Subvisible particulate matter | “Meets compendial requirements” | |
| 13. Appearance (Visible Particulates) | Appearance (Particles) | “Essentially free from visible particulates”[14] | |
| 14. Appearance | Appearance (Visual) | “White to off-white suspension” | |
| Identity | 15. Lipid identities | High-performance liquid chromatography with charged aerosol detector | Retention times and references are consistent (ALC-0159, ALC-3015, cholesterol, DSPC) |
| 16. Encoded RNA sequence identity | Reverse transcription polymerase chain reaction | Confirmed identity | |
| Product Purity | 17. RNA integrity | Capillary gel electrophoresis | ≥ 50% intact RNA |
| Adventitious Agents | 18. Bacterial endotoxins | Endotoxin (Limulus Amebocyte Lysate) | ≤ 12.5 endotoxin units per milliliter |
| 19. Sterility | Sterility | No growth detected | |
| 20. Container closure integrity | Dye incursion | Pass | |
Table 2: BNT162b2 Process Performance Qualification Specifications for Drug Substance Contained in the EC Contract (Nov 20, 2020)
| Process Performance Qualification Specifications for Drug Substance | |||
| Quality Attribute | Analytical Procedure | Acceptance Criteria | |
| Composition and Strength | 1. Content, RNA concentration | UV Spectroscopy | 2.00-2.50 milligram per milliliter |
| 2. pH | Potentiometry | 7.0 ± 0.5 | |
| 3. Coloration | Appearance | “Not more intensely colored than level 7 of the brown (B) color standard” | |
| 4. Clarity | Appearance | ≤ 6 nephelometric turbidity units | |
| Identity | 5. Encoded RNA sequence identity | Reverse transcription polymerase chain reaction | Confirmed identity |
| Product Purity | 6. 5’-Cap | Reversed-phase high-performance liquid chromatography | ≥ 50% 5’-Cap |
| 7. Poly (A) Tail | Droplet digital polymerase chain reaction | ≥ 70% Poly (A) Tail | |
| 8. RNA integrity | Capillary gel electrophoresis | ≥ 50% intact RNA | |
| Product-Related Impurities | 9. Residual DNA template | Quantitative polymerase chain reaction | ≤ 330 nanogram DNA per milligram RNA |
| 10. Residual double-stranded RNA | Immunoblot | ≤ 1000 picogram dsRNA per microgram RNA | |
| Adventitious Agents | 11. Bacterial endotoxins | Endotoxin (Limulus Amebocyte Lysate) | ≤ 12.5 endotoxin units per milliliter |
| 12. Bioburden | Bioburden | ≤ 1 colony forming unit per 10 milliliters | |
Governments Should Release the Information They Hold to Bolster Production, Advance Science
The manufacturing information released by the Italian public broadcaster can be helpful for scientists around the world. It also represents just a small fraction of the information about COVID vaccines that rich countries currently possess. As part of the regulatory process, manufacturers submit chemistry, manufacturing, and controls (CMC) data. This contains the vaccine recipe. In addition to specifications, it includes information about chemical characteristics; methods of manufacture, including raw material sources; flow charts of the manufacturing process, complete with a list of all tests performed at each step; process controls; drug substance batch records; and drug product master production records.[14] In the U.S., the Biomedical Advanced Research and Development Authority and the Department of Defense have access to this information under vaccine development contracts.[15]
In exchange for reasonable compensation to originator corporations, governments should release the information they hold.[16] Manufacturers in more than a dozen countries in Africa, Asia, and Latin America have expressed interest in producing mRNA vaccines.[17] Sharing information can help ramp up COVID vaccine production. Sharing information can also advance mRNA science by allowing scientists to quickly learn from each other’s work. Indeed, the development of safe and effective mRNA vaccines builds on decades of scientific discoveries across many different institutions.[18] Secrecy makes us less safe against this virus—and future pandemic threats.
References
[1] Advance Purchase Agreement between European Commission and Pfizer-BioNTech, SI2.838335 (Nov. 20, 2020), https://www.rai.it/dl/doc/2021/04/17/1618676600910_APA%20BioNTech%20Pfizer__.pdf (“the EC Contract”).
[2] Esclusiva Report: ecco i contratti “segreti” di Pfizer e Moderna per i vaccini anti-Covid, RAI (April 17 2021), https://www.rai.it/programmi/report/news/2021/04/Esclusiva-Report-ecco-i-contratti-segreti-di-Pfizer-e-Modena-per-i-vaccini-anti-Covid-b4edb1a2-3e84-48a4-b1eb-d02a1f7e2b4b.html
[3] Tweets by Member of La France Insoumise, Member of the Workers’ Party of Belgium and Wall Street Journal reporter. https://twitter.com/ManonAubryFr/status/1384043461137993733; https://twitter.com/BotengaM/status/1383470588908367873; https://twitter.com/bopanc/status/1384093800834899969.
[4] The EC Contract, pg. 60, https://www.rai.it/dl/doc/2021/04/17/1618676600910_APA%20BioNTech%20Pfizer__.pdf
[5] FDA, ICH, Q6B Specifications: Test Procedures and Acceptance Criteria for Biotechnological/Biological Products, (1999), https://www.fda.gov/regulatory-information/search-fda-guidance-documents/q6b-specifications-test-procedures-and-acceptance-criteria-biotechnologicalbiological-products
[6] FDA, Summary Basis for Regulatory Action, COMIRNATY, (Aug. 23 2021), https://www.fda.gov/media/151733/download, pg. 10-11.
[7] EMA, Comirnaty Assessment Report (Feb 19. 2021), https://www.ema.europa.eu/en/documents/assessment-report/comirnaty-epar-public-assessment-report_en.pdf
[8] FDA, Summary Basis for Regulatory Action, COMIRNATY, (Aug. 23 2021), https://www.fda.gov/media/151733/download, pg. 10-11.
[9] The EC Contract also lists procedure numbers and additional tests.
[10] The European Medicines Agency began its rolling review of the vaccine in June 2020. Pfizer likely had discussions with regulatory agencies about these initial criteria. Substantial variations from these criteria are unlikely since the vaccine received emergency use authorization in the U.S. and E.U. less than a month after the EC contract was signed in November. However, as part of its authorization, the EMA advised Pfizer that “the active substance and finished product specifications acceptance limits, should be reassessed and revised as appropriate, as further data becomes available from ongoing clinical trials and in line with manufacturing process capability and stability data of the product.” It also required Pfizer to introduce a new specification to control Poly(A) tail length and provide an update by July 2021. EMA, Comirnaty Assessment Report (Feb 19. 2021), https://www.ema.europa.eu/en/documents/assessment-report/comirnaty-epar-public-assessment-report_en.pdf. Pfizer initially used reversed-phase high-performance liquid chromatography to characterize the tail length. The EC Contract, pg. 63. https://www.rai.it/dl/doc/2021/04/17/1618676600910_APA%20BioNTech%20Pfizer__.pdf.
[11] The order of the attributes in the original contract and the FDA table also appear similar. However, we have sorted the attributes in reverse order in this analysis.
[12] The routine release specifications reported by the FDA appear to call this “vial content (volume)”. FDA, Summary Basis for Regulatory Action, COMIRNATY, (Aug. 23 2021), https://www.fda.gov/media/151733/download, pg. 10.
[13] The routine release specifications reported by the FDA instead say “may contain white to off-white opaque, amorphous particles.” Id.
[14] Guidance for Industry: Content and Format of Chemistry Manufacturing and Controls Information and Establishment Description Information for a Vaccine or Related Product, FDA (Jan. 1999), https://www.fda.gov/media/73614/download
[15] COVID-19 Vaccine Contract between Department of Defense and Pfizer (July 21 2020), https://www.hhs.gov/sites/default/files/pfizer-inc-covid-19-vaccine-contract.pdf. (“Pfizer shall provide copies of EUA and BLA filings, as well as interim and final data updates from clinical studies in a format determined by Pfizer”). Pfizer was also required to provide a manufacturing development plan. Pg 16.
[16] Zain Rizvi, Jishian Ravinthiran, Amy Kapczynski, Sharing The Knowledge: How President Joe Biden Can Use The Defense Production Act To End The Pandemic Worldwide, Health Affairs Blog (August 6, 2021), https://www.healthaffairs.org/do/10.1377/hblog20210804.101816/full/
[17] COVAX Manufacturing Taskforce Slides, May 12, 2021, https://www.who.int/publications/m/item/covax-manufacturing-taskforce
[18] Hou et al., Lipid nanoparticles for mRNA delivery, Nature Reviews Materials (Aug. 10 2021), https://www.nature.com/articles/s41578-021-00358-0 (Figure 1).